Clin Endosc.
2018 Nov;51(6):584-586. 10.5946/ce.2018.038.
Gastric Ulceration and Bleeding with Hemodynamic Instability Caused by an Intragastric Balloon for Weight Loss
- Affiliations
-
- 1Department of Internal Medicine, Texas Tech University Health Sciences Center, Lubbock, TX, USA. kenneth.nugent@ttuhsc.edu
- KMID: 2430402
- DOI: http://doi.org/10.5946/ce.2018.038
Abstract
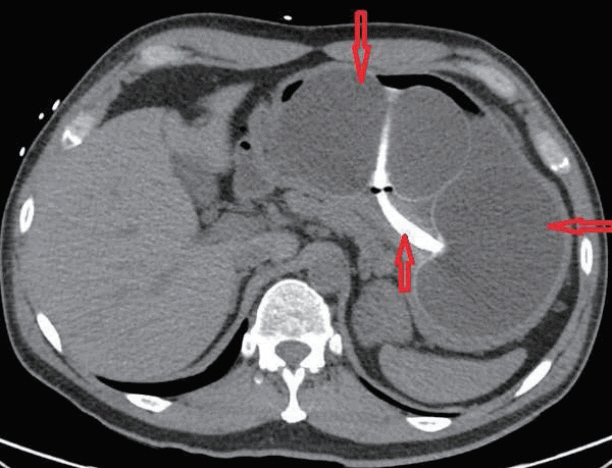
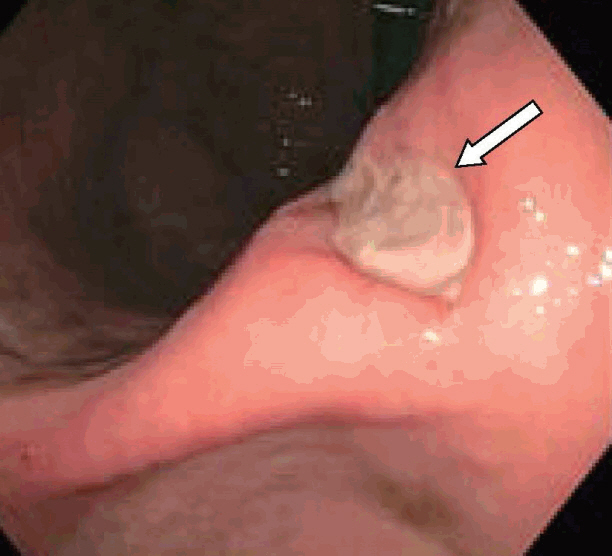
- Obesity in the United States is a medical crisis with many people attempting to lose weight with caloric restriction. Some patients choose minimally invasive weight loss solutions, such as intragastric balloon systems. These balloon systems were approved by the Federal Drug Administration (FDA) in 2015-2016 and have been considered safe, with minimal side effects. We report a patient with a two-day history of melena, abdominal pain, hypotension, and syncope which developed five months after placement of an intragastric balloon. Esophagogastroduodenoscopy with balloon removal revealed a small 8-mm gastric ulcer in the incisura. This gastric ulcer probably developed secondary to mechanical compression of the stomach mucosa by the gastric balloon which contained 900 mL of saline. The FDA is now investigating five deaths since 2016 associated with these second-generation balloons. Clinicians should be aware of these complications when evaluating patients with gastrointestinal complications, such as bleeding.
MeSH Terms
Figure
Reference
-
1. Kumar N, Sullivan S, Thompson CC. The role of endoscopic therapy in obesity management: intragastric balloons and aspiration therapy. Diabetes Metab Syndr Obes. 2017; 10:311–316.
Article2. Papademetriou M, Popov V. Intragastric balloons in clinical practice. Gastrointest Endosc Clin N Am. 2017; 27:245–256.
Article3. Yoo IK, Chun HJ, Jeen YT. Gastric perforation caused by an intragastric balloon: endoscopic findings. Clin Endosc. 2017; 50:602–604.
Article4. U.S. Food and Drug Administration. Liquid-filled intragastric balloon systems: letter to healthcare providers - potential risks [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;c2017. [updated 2018 Jan 8; cited 2017 Oct 10]. Available from: https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm570916.htm.5. Ponce J, Woodman G, Swain J, et al. The REDUCE pivotal trial: a prospective, randomized controlled pivotal trial of a dual intragastric balloon for the treatment of obesity. Surg Obes Relat Dis. 2015; 11:874–881.
Article6. Vyas D, Deshpande K, Pandya Y. Advances in endoscopic balloon therapy for weight loss and its limitations. World J Gastroenterol. 2017; 23:7813–7817.
Article7. ReShape Medical. ReShape® integrated dual balloon system instructions for use [Internet]. San Clemente (CA): ReShape Medical;c2015. [updated 2015 Dec 15; cited 2017 Oct 10]. Available from: https://reshapeready.com/wp-content/uploads/2015/07/ReShape_Instructions_For_Use.pdf.8. Al Shammari NM, Alshammari AS, Alkandari MA, Abdulsalam AJ. Migration of an intragastric balloon: a case report. Int J Surg Case Rep. 2016; 27:10–12.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Various Intragastric Balloons Under Clinical Investigation
- Short-term Outcomes of Intragastric Balloon Placement for Obesity Treatment
- Gastric Perforation Caused by an Intragastric Balloon: Endoscopic Findings
- Transarterial Embolization of Massive Gastric Ulcer Bleeding in Gastrostomy Patients Caused by a Balloon Replacement Tube: A Case Report
- A Case of Severe Gastric Ulcer Bleeding after Exchange for Replacement Balloon Gastrostomy Tube in Percutaneous Endoscopic Gastrostomy