Korean J Physiol Pharmacol.
2018 Nov;22(6):649-660. 10.4196/kjpp.2018.22.6.649.
Effect of carbamazepine on tetrodotoxin-resistant Na⺠channels in trigeminal ganglion neurons innervating to the dura
- Affiliations
-
- 1Department of Pharmacology, School of Dentistry, Kyungpook National University, Daegu 41940, Korea. jis7619@knu.ac.kr
- 2Brain Science & Engineering Institute, Kyungpook National University, Daegu 41940, Korea.
- 3Department of Pharmacology, School of Medicine, Kyungpook National University, Daegu 41405, Korea.
- KMID: 2430087
- DOI: http://doi.org/10.4196/kjpp.2018.22.6.649
Abstract
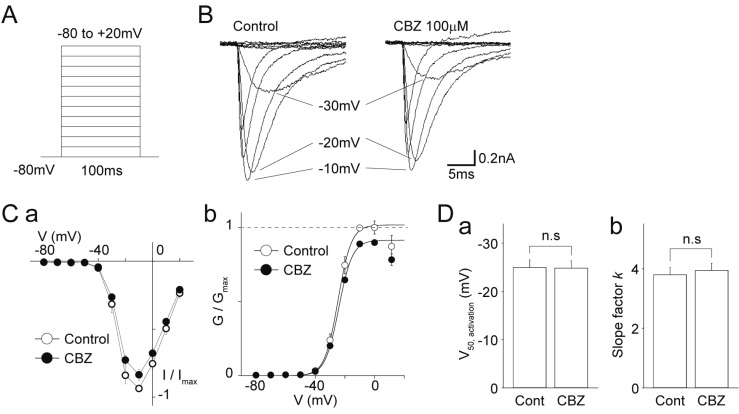
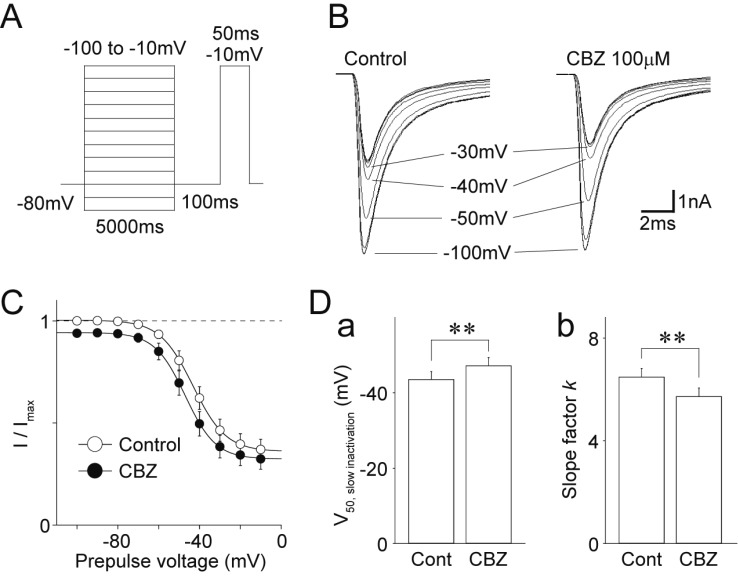
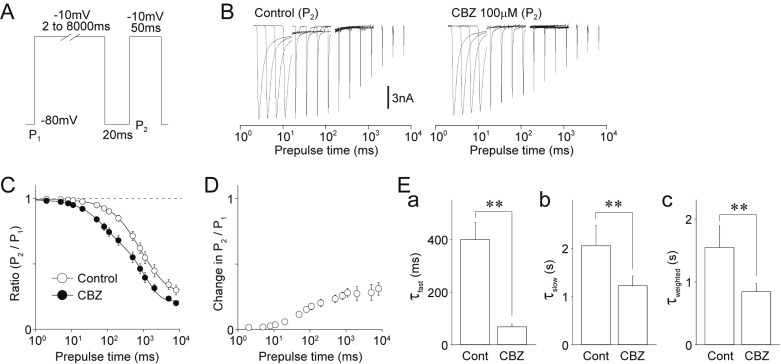
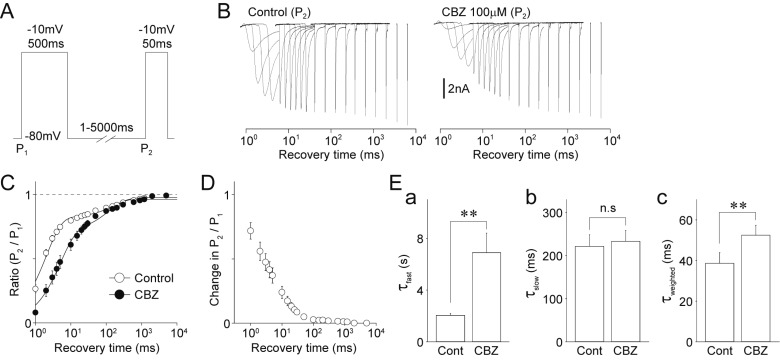
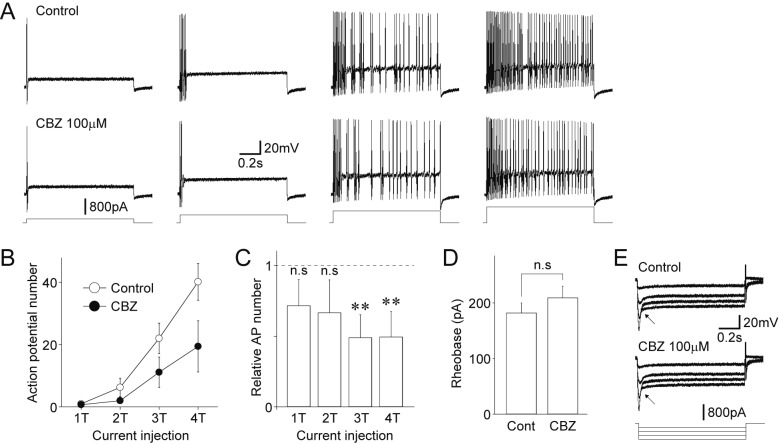
- Migraine is a neurological disorder characterized by recurrent and disabling severe headaches. Although several anticonvulsant drugs that block voltage-dependent Na⺠channels are widely used for migraine, far less is known about the therapeutic actions of carbamazepine on migraine. In the present study, therefore, we characterized the effects of carbamazepine on tetrodotoxin-resistant (TTX-R) Na⺠channels in acutely isolated rat dural afferent neurons, which were identified by the fluorescent dye DiI. The TTX-R Na⺠currents were measured in medium-sized DiIpositive neurons using the whole-cell patch clamp technique in the voltage-clamp mode. While carbamazepine had little effect on the peak amplitude of transient Na⺠currents, it strongly inhibited steady-state currents of transient as well as persistent Na⺠currents in a concentration-dependent manner. Carbamazepine had only minor effects on the voltage-activation relationship, the voltage-inactivation relationship, and the use-dependent inhibition of TTX-R Na⺠channels. However, carbamazepine changed the inactivation kinetics of TTX-R Na⺠channels, significantly accelerating the development of inactivation and delaying the recovery from inactivation. In the current-clamp mode, carbamazepine decreased the number of action potentials without changing the action potential threshold. Given that the sensitization of dural afferent neurons by inflammatory mediators triggers acute migraine headaches and that inflammatory mediators potentiate TTX-R Na⺠currents, the present results suggest that carbamazepine may be useful for the treatment of migraine headaches.
Keyword
MeSH Terms
Figure
Reference
-
1. Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med. 2007; 13:39–44. PMID: 17141570.
Article2. Diener HC, Dodick DW, Goadsby PJ, Lipton RB, Olesen J, Silberstein SD. Chronic migraine--classification, characteristics and treatment. Nat Rev Neurol. 2012; 8:162–171. PMID: 22331030.
Article3. Ferrari MD. The economic burden of migraine to society. Pharmacoeconomics. 1998; 13:667–676. PMID: 10179702.
Article4. Michel P, Dartigues JF, Lindoulsi A, Henry P. Loss of productivity and quality of life in migraine sufferers among French workers: results from the GAZEL cohort. Headache. 1997; 37:71–78. PMID: 9074290.
Article5. Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, Sándor PS. EFNS guideline on the drug treatment of migraine--revised report of an EFNS task force. Eur J Neurol. 2009; 16:968–981. PMID: 19708964.6. Malik SN, Hopkins M, Young WB, Silberstein SD. Acute migraine treatment: patterns of use and satisfaction in a clinical population. Headache. 2006; 46:773–780. PMID: 16643580.
Article7. Silberstein SD, Marmura MJ. Acute migraine treatment. Headache. 2015; 55:1–2.
Article8. Silberstein SD, Goadsby PJ. Migraine: preventive treatment. Cephalalgia. 2002; 22:491–512. PMID: 12230591.
Article9. Silberstein SD. Preventive treatment of migraine. Trends Pharmacol Sci. 2006; 27:410–415. PMID: 16820222.
Article10. Sidhu HS, Sadhotra A. Current status of the new antiepileptic drugs in chronic pain. Front Pharmacol. 2016; 7:276. PMID: 27610084.
Article11. Rogawski M. Common pathophysiologic mechanisms in migraine and epilepsy. Arch Neurol. 2008; 65:709–714. PMID: 18541791.
Article12. Linde M, Mulleners WM, Chronicle EP, McCrory DC. Topiramate for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013; (6):CD010610. PMID: 23797676.
Article13. Linde M, Mulleners WM, Chronicle EP, McCrory DC. Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013; (6):CD010611. PMID: 23797677.
Article14. Rompel H, Bauermeister PW. Aetiology of migraine and prevention with carbamazepine (Tegretol): results of a double-blind, cross-over study. S Afr Med J. 1970; 44:75–80. PMID: 4905910.15. Steiner TJ, Findley LJ, Yuen AW. Lamotrigine versus placebo in the prophylaxis of migraine with and without aura. Cephalalgia. 1997; 17:109–112. PMID: 9137848.
Article16. Silberstein S, Saper J, Berenson F, Somogyi M, McCague K, D'Souza J. Oxcarbazepine in migraine headache: a double-blind, randomized, placebo-controlled study. Neurology. 2008; 70:548–555. PMID: 18268247.
Article17. Gupta P, Singh S, Goyal V, Shukla G, Behari M. Low-dose topiramate versus lamotrigine in migraine prophylaxis (the Lotolamp study). Headache. 2007; 47:402–412. PMID: 17371357.
Article18. Tanelian DL, Brose WG. Neuropathic pain can be relieved by drugs that are use-dependent sodium channel blockers: lidocaine, carbamazepine, and mexiletine. Anesthesiology. 1991; 74:949–951. PMID: 1850581.19. Baker KA, Taylor JW, Lilly GE. Treatment of trigeminal neuralgia: use of baclofen in combination with carbamazepine. Clin Pharm. 1985; 4:93–96. PMID: 3971692.20. Sidebottom A, Maxwell S. The medical and surgical management of trigeminal neuralgia. J Clin Pharm Ther. 1995; 20:31–35. PMID: 7775611.
Article21. Backonja MM. Use of anticonvulsants for treatment of neuropathic pain. Neurology. 2002; 59(5 Suppl 2):S14–S17. PMID: 12221151.
Article22. Rush AM, Elliott JR. Phenytoin and carbamazepine: differential inhibition of sodium currents in small cells from adult rat dorsal root ganglia. Neurosci Lett. 1997; 226:95–98. PMID: 9159498.
Article23. Bräu ME, Dreimann M, Olschewski A, Vogel W, Hempelmann G. Effect of drugs used for neuropathic pain management on tetrodotoxin-resistant Na+ currents in rat sensory neurons. Anesthesiology. 2001; 94:137–144. PMID: 11135733.24. Harriott AM, Gold MS. Electrophysiological properties of dural afferents in the absence and presence of inflammatory mediators. J Neurophysiol. 2009; 101:3126–3134. PMID: 19339455.
Article25. Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989; 103:56–63. PMID: 2476693.
Article26. Galletti F, Cupini LM, Corbelli I, Calabresi P, Sarchielli P. Pathophysiological basis of migraine prophylaxis. Prog Neurobiol. 2009; 89:176–192. PMID: 19654035.
Article27. Chiossi L, Negro A, Capi M, Lionetto L, Martelletti P. Sodium channel antagonists for the treatment of migraine. Expert Opin Pharmacother. 2014; 15:1697–1706. PMID: 24941134.
Article28. Lampl C, Katsarava Z, Diener HC, Limmroth V. Lamotrigine reduces migraine aura and migraine attacks in patients with migraine with aura. J Neurol Neurosurg Psychiatry. 2005; 76:1730–1732. PMID: 16291905.
Article29. Drake ME Jr, Greathouse NI, Renner JB, Armentbright AD. Open-label zonisamide for refractory migraine. Clin Neuropharmacol. 2004; 27:278–280. PMID: 15613932.
Article30. Mohammadianinejad SE, Abbasi V, Sajedi SA, Majdinasab N, Abdollahi F, Hajmanouchehri R, Faraji A. Zonisamide versus topiramate in migraine prophylaxis: a double-blind randomized clinical trial. Clin Neuropharmacol. 2011; 34:174–177. PMID: 21738025.31. Johannessen CU. Mechanisms of action of valproate: a commentatory. Neurochem Int. 2000; 37:103–110. PMID: 10812195.
Article32. Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000; 41(Suppl 1):S3–S9.
Article33. Schmidt D, Elger CE. What is the evidence that oxcarbazepine and carbamazepine are distinctly different antiepileptic drugs? Epilepsy Behav. 2004; 5:627–635. PMID: 15380112.
Article34. Dailey JW, Reith ME, Yan QS, Li MY, Jobe PC. Carbamazepine increases extracellular serotonin concentration: lack of antagonism by tetrodotoxin or zero Ca2+. Eur J Pharmacol. 1997; 328:153–162. PMID: 9218697.35. Dailey JW, Reith ME, Steidley KR, Milbrandt JC, Jobe PC. Carbamazepine-induced release of serotonin from rat hippocampus in vitro. Epilepsia. 1998; 39:1054–1063. PMID: 9776325.
Article36. Kawata Y, Okada M, Murakami T, Kamata A, Zhu G, Kaneko S. Pharmacological discrimination between effects of carbamazepine on hippocampal basal, Ca2+- and K+-evoked serotonin release. Br J Pharmacol. 2001; 133:557–567. PMID: 11399673.37. Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997; 340:249–258. PMID: 9537821.
Article38. Xu XM, Yang C, Liu Y, Dong MX, Zou DZ, Wei YD. Efficacy and feasibility of antidepressants for the prevention of migraine in adults: a meta-analysis. Eur J Neurol. 2017; 24:1022–1031. PMID: 28557171.
Article39. Silberstein SD. Migraine pathophysiology and its clinical implications. Cephalalgia. 2004; 24(Suppl 2):2–7.
Article40. Benemei S, Nicoletti P, Capone JG, Geppetti P. CGRP receptors in the control of pain and inflammation. Curr Opin Pharmacol. 2009; 9:9–14. PMID: 19157980.
Article41. Bolay H, Reuter U, Dunn AK, Huang Z, Boas DA, Moskowitz MA. Intrinsic brain activity triggers trigeminal meningeal afferents in a migraine model. Nat Med. 2002; 8:136–142. PMID: 11821897.
Article42. Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci USA. 1996; 93:1108–1112. PMID: 8577723.43. Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998; 18:10345–10355. PMID: 9852572.44. Nakamura M, Jang IS. Characterization of dural afferent neurons innervating cranial blood vessels within the dura in rats. Brain Res. 2018; 1696:91–102. PMID: 29886250.
Article45. Schneider H, Stenzel E. [Carbamazepine--daily course in the serum during long term medication]. Bibl Psychiatr. 1975; (151):32–42. PMID: 1156346.46. Bertilsson L. Clinical pharmacokinetics of carbamazepine. Clin Pharmacokinet. 1978; 3:128–143. PMID: 346287.
Article47. Taverna S, Mantegazza M, Franceschetti S, Avanzini G. Valproate selectively reduces the persistent fraction of Na+ current in neocortical neurons. Epilepsy Res. 1998; 32:304–308. PMID: 9761329.48. Taverna S, Sancini G, Mantegazza M, Franceschetti S, Avanzini G. Inhibition of transient and persistent Na+ current fractions by the new anticonvulsant topiramate. J Pharmacol Exp Ther. 1999; 288:960–968. PMID: 10027832.49. Stafstrom CE. Persistent sodium current and its role in epilepsy. Epilepsy Curr. 2007; 7:15–22. PMID: 17304346.
Article50. Hains BC, Waxman SG. Sodium channel expression and the molecular pathophysiology of pain after SCI. Prog Brain Res. 2007; 161:195–203. PMID: 17618978.
Article51. Hur YK, Choi IS, Cho JH, Park EJ, Choi JK, Choi BJ, Jang IS. Effects of carbamazepine and amitriptyline on tetrodotoxinresistant Na+ channels in immature rat trigeminal ganglion neurons. Arch Pharm Res. 2008; 31:178–182. PMID: 18365687.52. Willow M, Gonoi T, Catteral W. Voltage clamp analysis of the inhibitory actions of diphenyihydantoin and carbamazepine on voltage-sensitive sodium channels in neuroblastoma cells. J Mol Pharmacol. 1985; 27:549–558.53. Singh JN, Jain G, Ramarao P, Sharma SS. Inhibition of sodium current by carbamazepine in dorsal root ganglion neurons in vitro. Indian J Physiol Pharmacol. 2009; 53:147–154. PMID: 20112818.54. Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996; 58:349–362. PMID: 8815799.
Article55. Bennett BD, Callaway JC, Wilson CJ. Intrinsic membrane properties underlying spontaneous tonic firing in neostriatal cholinergic interneurons. J Neurosci. 2000; 20:8493–8503. PMID: 11069957.
Article56. Taddese A, Bean BP. Subthreshold sodium current from rapidly inactivating sodium channels drives spontaneous firing of tuberomammillary neurons. Neuron. 2002; 33:587–600. PMID: 11856532.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution of ion channels in trigeminal ganglion neuron of rat
- Effects of Fluoxetine on Sodium Currents in Rat Sensory Neurons
- Role of neuron and non-neuronal cell communication in persistent orofacial pain
- Response to Capsaicin and Changes in Sodium Currents in Dorsal Root Ganglion Neurons of Neuropathic Rats
- Alcohol Neurolyisis for the Treatment of Tregeminal Neuralgia