Transl Clin Pharmacol.
2018 Dec;26(4):145-149. 10.12793/tcp.2018.26.4.145.
Five years of the CiPA project (2013–2018): what did we learn?
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Seoul St. Mary's Hospital, Seoul 06591, Korea. yimds@catholic.ac.kr
- 2PIPET (Pharmacometrics Institute for Practical Education and Training), College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- KMID: 2429508
- DOI: http://doi.org/10.12793/tcp.2018.26.4.145
Abstract
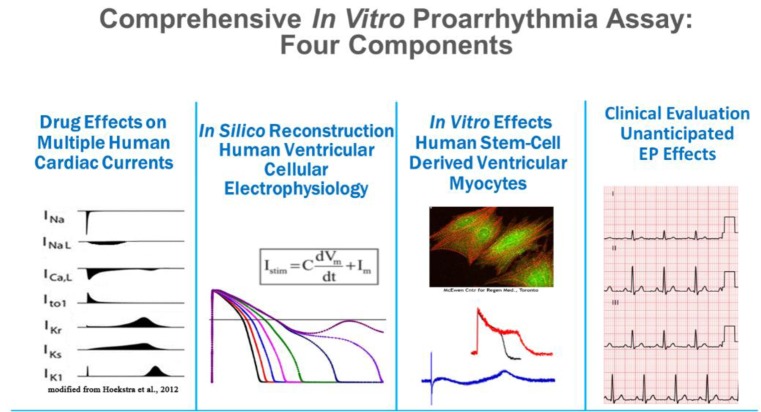
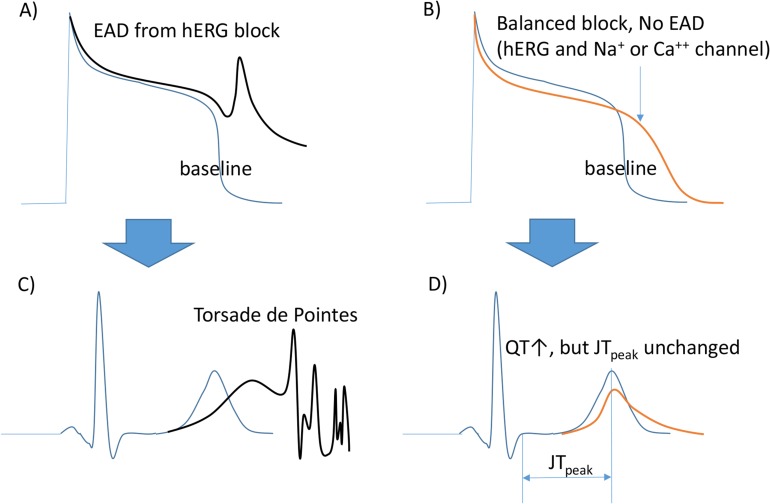
- Cases of drug-induced QT prolongation and sudden cardiac deaths resulted in market withdrawal of many drugs and world-wide regulatory changes through accepting the ICH guidelines E14 and S7B. However, because the guidelines were not comprehensive enough to cover the electrophysiological changes by drug-induced cardiac ion channel blocking, CiPA was initiated by experts in governments and academia in the USA, Europe, and Japan in 2013. Five years have passed since the launch of the CiPA initiative that aimed to improve the current ICH guidelines. This report reviews the current achievements of the CiPA initiative and explores unresolved issues.
Keyword
MeSH Terms
Figure
Reference
-
1. Brown A. Drugs, hERG and sudden death. Cell Calcium. 2004; 35:543–547. DOI: 10.1016/j.ceca.2004.01.008. PMID: 15110144.
Article2. Delpon E, Valenzuela C, Tamargo J. Blockade of cardiac potassium and other channels by antihistamines. Drug Saf. 1999; 21(Suppl 1):11–18. discussion 81-17. DOI: 10.2165/00002018-199921001-00003. PMID: 10597864.3. Shah RR. Drugs, QTc Interval prolongation and final ICH E14 guideline. Drug Saf. 2005; 28:1009–1028. DOI: 10.2165/00002018-200528110-00003. PMID: 16231954.
Article4. Darpo B. The thorough QT/QTc study 4 years after the implementation of the ICH E14 guidance. Br J Pharmacol. 2010; 159:49–57. DOI: 10.1111/j.1476-5381.2009.00487.x. PMID: 19922536.
Article5. Redfern WS, Carlsson L, Davis AS, Lynch WG, MacKenzie I, Palethorpe S, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: evidence for a provisional safety margin in drug development. Cardiovasc Res. 2003; 58:32–45. PMID: 12667944.
Article6. Li Z, Ridder BJ, Han X, Wu WW, Sheng J, Tran PN, et al. Assessment of an In Silico Mechanistic Model for Proarrhythmia Risk Prediction Under the Ci PA Initiative. Clin Pharmacol Ther. 2018; DOI: 10.1002/cpt.1184.7. O'Hara T, Virag L, Varro A, Rudy Y. Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation. PLoS Comput Biol. 2011; 7:e1002061. DOI: 10.1371/journal.pcbi.1002061.PMID: 21637795.8. Li Z, Dutta S, Sheng J, Tran PN, Wu W, Chang K, et al. Improving the In Silico Assessment of Proarrhythmia Risk by Combining hERG (Human Ether-a-go-go-Related Gene) Channel-Drug Binding Kinetics and Multichannel Pharmacology. Circ Arrhythm Electrophysiol. 2017; 10:e004628. DOI: 10.1161/CIRCEP.116.004628. PMID: 28202629.
Article9. Crumb WJ Jr, Vicente J, Johannesen L, Strauss DG. An evaluation of 30 clinical drugs against the comprehensive in vitro proarrhythmia assay (CiPA) proposed ion channel panel. J Pharmacol Toxicol Methods. 2016; 81:251–262. DOI: 10.1016/j.vascn.2016.03.009. PMID: 27060526.
Article10. Dutta S, Chang KC, Beattie KA, Sheng J, Tran PN, Wu WW, et al. Optimization of an In silico Cardiac Cell Model for Proarrhythmia Risk Assessment. Front Physiol. 2017; 8:616. DOI: 10.3389/fphys.2017.00616. PMID: 28878692.
Article11. Elkins RC, Davies MR, Brough SJ, Gavaghan DJ, Cui Y, Abi-Gerges N, et al. Variability in high-throughput ion-channel screening data and consequences for cardiac safety assessment. J Pharmacol Toxicol Methods. 2013; 68:112–122. DOI: 10.1016/j.vascn.2013.04.007. PMID: 23651875.
Article12. Mistry HB. Complexity vs. Simplicity: The Winner Is? Clin Pharmacol Ther. 2017; 101:326. DOI: 10.1002/cpt.503. PMID: 27617708.
Article13. Mistry HB. Complex versus simple models: ion-channel cardiac toxicity prediction. PeerJ. 2018; 6:e4352. DOI: 10.7717/peerj.4352. PMID: 29423349.
Article14. Blinova K, Stohlman J, Vicente J, Chan D, Johannesen L, Hortigon-Vinagre MP, et al. Comprehensive Translational Assessment of Human-Induced Pluripotent Stem Cell Derived Cardiomyocytes for Evaluating Drug-Induced Arrhythmias. Toxicol Sci. 2017; 155:234–247. DOI: 10.1093/toxsci/kfw200. PMID: 27701120.
Article15. Johannesen L, Vicente J, Mason JW, Sanabria C, Waite-Labott K, Hong M, et al. Differentiating drug-induced multichannel block on the electrocardiogram: randomized study of dofetilide, quinidine, ranolazine, and verapamil. Clin Pharmacol Ther. 2014; 96:549–558. DOI: 10.1038/clpt.2014.155. PMID: 25054430.
Article16. Vicente J, Zusterzeel R, Johannesen L, Mason J, Sager P, Patel V, et al. Mechanistic Model-Informed Proarrhythmic Risk Assessment of Drugs: Review of the “CiPA” Initiative and Design of a Prospective Clinical Validation Study. Clin Pharmacol Ther. 2018; 103:54–66. DOI: 10.1002/cpt.896. PMID: 28986934.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Generalized Xerotic Eczema in a Patient with Congenital Insensitivity to Pain with Anhidrosis
- Anesthetic Management in a Patient with Congenital Insensitivity to Pain with Anhidrosis: A case report
- Introduction to in silico model for proarrhythmic risk assessment under the CiPA initiative
- Anesthetic Management for a Patient with Congenital Insensitivity to Pain with Anhidrosis (CIPA): A case report
- A Case of Congenital Insensitivity to Pain with Anhidrosis