Blood Res.
2018 Sep;53(3):210-217. 10.5045/br.2018.53.3.210.
Effectiveness of L-asparaginase-based regimens compared to anthracycline-based regimens in newly diagnosed extranodal NK/T-cell lymphoma, nasal type: a single Mexican center experience
- Affiliations
-
- 1Department of Hematology-Oncology, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, México City, México. al_avila_milord@icloud.com
- KMID: 2429320
- DOI: http://doi.org/10.5045/br.2018.53.3.210
Abstract
- BACKGROUND
Extranodal NK/T-cell lymphoma, nasal type (ENKTCL) has a high prevalence in Asia and Latin American countries, such as Mexico, where it encompasses 40% of all T-cell non-Hodgkin lymphomas. Historically, responses to anthracycline-based therapies have been disappointing. Since data about the effectiveness of L-asparaginase-based regimens in Mexico are limited, we compared both therapies in our center.
METHODS
We performed a retrospective cohort of patients with newly diagnosed ENKTCL, who were divided into two groups for treatment and analysis (group 1: L-asparaginase-based regimen and group 2: anthracycline-based regimen) between 2001 and 2016.
RESULTS
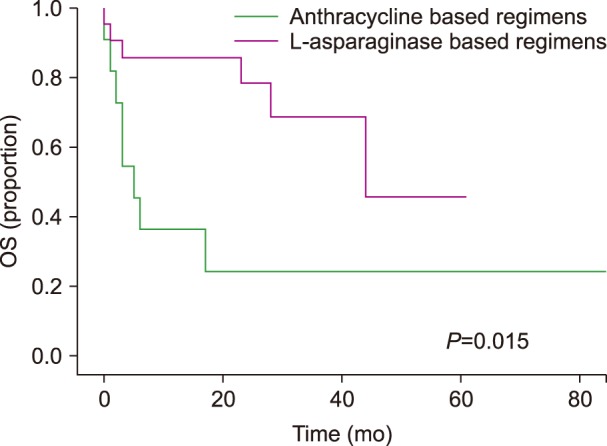
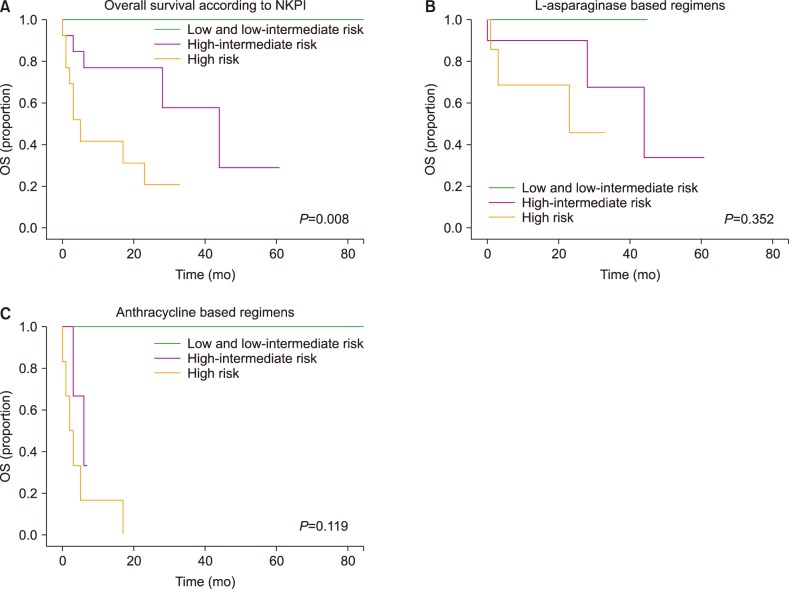
Of 36 patients with newly-diagnosed ENKTCL, 33 received at least one cycle of chemotherapy (22 in group 1 and 11 in group 2). Over a median follow-up interval of 17 months (range, 0-167), a complete response (CR) was observed in 45.5% of patients in group 1, compared to 27% of group 2 (P=0.45). Progression was more frequently observed in group 2 than in group 1 (54.5% vs. 18.4%, P=0.04). The median overall survival (OS) was 44 months in group 1, compared to 5 months in group 2 (P=0.012). The multivariate analysis showed that failure to achieve a CR after first-line therapy was the only significant factor for OS (HR, 3.04; 95% CI, 1.4-6.5; P=0.005).
CONCLUSION
L-asparaginase-based regimens for patients with newly-diagnosed ENKTCL confer a survival advantage over anthracycline-based regimens.
Keyword
MeSH Terms
Figure
Reference
-
1. Vose J, Armitage J, Weisenburger D. International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008; 26:4124–4130. PMID: 18626005.2. Laurini JA, Perry AM, Boilesen E, et al. Classification of non-Hodgkin lymphoma in Central and South America: a review of 1028 cases. Blood. 2012; 120:4795–4801. PMID: 23086753.
Article3. Avilés A. Nasal NK/T-cell lymphoma. A comparative analysis of a mexican population with the other populations of Latin-America. Mediterr J Hematol Infect Dis. 2015; 7:e2015052. PMID: 26401241.4. Kim WS, Song SY, Ahn YC, et al. CHOP followed by involved field radiation: is it optimal for localized nasal natural killer/T-cell lymphoma? Ann Oncol. 2001; 12:349–352. PMID: 11332147.
Article5. Li YX, Yao B, Jin J, et al. Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol. 2006; 24:181–189. PMID: 16382127.
Article6. Ma HH, Qian LT, Pan HF, et al. Treatment outcome of radiotherapy alone versus radiochemotherapy in early stage nasal natural killer/T-cell lymphoma. Med Oncol. 2010; 27:798–806. PMID: 19685292.
Article7. Cheung MM, Chan JK, Lau WH, et al. Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol. 1998; 16:70–77. PMID: 9440725.
Article8. Chim CS, Ma SY, Au WY, et al. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood. 2004; 103:216–221. PMID: 12933580.
Article9. Yamaguchi M, Kita K, Miwa H, et al. Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer. 1995; 76:2351–2356. PMID: 8635042.
Article10. Tse E, Kwong YL. How I treat NK/T-cell lymphomas. Blood. 2013; 121:4997–5005. PMID: 23652805.
Article11. Yong W, Zheng W, Zhang Y, et al. L-asparaginase-based regimen in the treatment of refractory midline nasal/nasal-type T/NK-cell lymphoma. Int J Hematol. 2003; 78:163–167. PMID: 12953813.
Article12. Yong W, Zheng W, Zhu J, et al. L-asparaginase in the treatment of refractory and relapsed extranodal NK/T-cell lymphoma, nasal type. Ann Hematol. 2009; 88:647–652. PMID: 19107482.
Article13. Tse E, Kwong YL. Diagnosis and management of extranodal NK/T cell lymphoma nasal type. Expert Rev Hematol. 2016; 9:861–871. PMID: 27347812.
Article14. Sobrevilla-Calvo P, Meneses A, Alfaro P, Bares JP, Amador J, Reynoso EE. Radiotherapy compared to chemotherapy as initial treatment of angiocentric centrofacial lymphoma (polymorphic reticulosis). Acta Oncol. 1993; 32:69–72. PMID: 8466767.
Article15. Avilés A, Díaz NR, Neri N, Cleto S, Talavera A. Angiocentric nasal T/natural killer cell lymphoma: a single centre study of prognostic factors in 108 patients. Clin Lab Haematol. 2000; 22:215–220. PMID: 11012633.
Article16. Avilés A, Neri N, Fernández R, Huerta-Guzmán J, Nambo MJ. Combined therapy in untreated patients improves outcome in nasal NK/T lymphoma: results of a clinical trial. Med Oncol. 2013; 30:637. PMID: 23797771.
Article17. Chan J, Quintanilla-Martínez L, Ferry J, Peh S. Extranodal NK/T cell lymphoma, nasal type. In : Swerdlow SH, Campo E, Harris NL, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France: IARC Press;2008. p. 285–288.18. Jiang M, Zhang H, Jiang Y, et al. Phase 2 trial of “sandwich” L-asparaginase, vincristine, and prednisone chemotherapy with radiotherapy in newly diagnosed, stage IE to IIE, nasal type, extranodal natural killer/T-cell lymphoma. Cancer. 2012; 118:3294–3301. PMID: 22139825.19. Jaccard A, Gachard N, Marin B, et al. Efficacy of L-asparaginase with methotrexate and dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. Blood. 2011; 117:1834–1839. PMID: 21123825.
Article20. McKelvey EM, Gottlieb JA, Wilson HE, et al. Hydroxyldaunomycin (Adriamycin) combination chemotherapy in malignant lymphoma. Cancer. 1976; 38:1484–1493. PMID: 791473.
Article21. Pfreundschuh M, Trümper L, Kloess M, et al. Two-weekly or 3-weekly CHOP chemotherapy with or without etoposide for the treatment of elderly patients with aggressive lymphomas: results of the NHL-B2 trial of the DSHNHL. Blood. 2004; 104:634–641. PMID: 15016643.
Article22. International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993; 329:987–994. PMID: 8141877.23. Lee J, Suh C, Park YH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006; 24:612–618. PMID: 16380410.
Article24. Kim SJ, Yoon DH, Jaccard A, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016; 17:389–400. PMID: 26873565.
Article25. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–3068. PMID: 25113753.
Article26. Qi S, Yahalom J, Hsu M, et al. Encouraging experience in the treatment of nasal type extra-nodal NK/T-cell lymphoma in a non-Asian population. Leuk Lymphoma. 2016; 57:2575–2583. PMID: 27183991.
Article27. Huang L, Yuan B, Wu H, et al. Comparative study of L-asparaginase-based LOP regimen over CHOP regimen before radiotherapy for stage IIE extranodal nasal type NK/T cell lymphoma: a study of 2 centers. Clin Lymphoma Myeloma Leuk. 2017; 17:152–158. PMID: 28215935.
Article28. Au WY, Weisenburger DD, Intragumtornchai T, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009; 113:3931–3937. PMID: 19029440.
Article29. Makita S, Tobinai K. Clinical features and current optimal management of natural killer/T-cell lymphoma. Hematol Oncol Clin North Am. 2017; 31:239–253. PMID: 28340876.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extranodal NK/T cell lymphoma
- Extranodal NK/T Cell Lymphoma, Nasal Type that Occurred in Patients with Atrophic Rhinitis
- Circulating Low Absolute CD4⺠T Cell Counts May Predict Poor Prognosis in Extranodal NK/T-Cell Lymphoma Patients Treating with Pegaspargase-Based Chemotherapy
- A Case of Extranodal NK/T-cell Lymphoma at the Base of Tongue
- A Case of Extranodall NK/T-cell Lymphoma, Nasal type