Cancer Res Treat.
2019 Jan;51(1):368-377. 10.4143/crt.2018.010.
Circulating Low Absolute CD4⺠T Cell Counts May Predict Poor Prognosis in Extranodal NK/T-Cell Lymphoma Patients Treating with Pegaspargase-Based Chemotherapy
- Affiliations
-
- 1Department of Hematology, the First Affiliated Hospital of Nanjing Medical University, Jiangsu Province Hospital, Nanjing, China. lijianyonglm@medmail.com.cn, xuwei10000@hotmail.com
- 2Key Laboratory of Hematology of Nanjing Medical University, Nanjing, China.
- 3Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing, China.
- 4Department of Hematology, Affiliated Hospital of Nantong University, Nantong, China.
- KMID: 2437627
- DOI: http://doi.org/10.4143/crt.2018.010
Abstract
- PURPOSE
Extranodal natural killer/T-cell lymphoma, nasal type (ENKTL) is a rare subtype of non-Hodgkin lymphoma, and asparaginase-based regimens are the best first-line treatments. Data on the role of specific circulating lymphocyte subsets in the progression of ENKTL are limited. The aim of this study was to investigate the clinical correlation and distribution of circulating absolute CD4+ T-cell counts (ACD4Cs) in ENKTL.
MATERIALS AND METHODS
We retrospectively searched medical records for 70 newly diagnosed ENKTL patients treated with pegaspargase-based regimens. Comparison of ACD4Cs as a continuous parameter in different groups was calculated. Univariate and multivariate analyses were used to assess prognostic factors for overall survival (OS) and progression-free survival (PFS).
RESULTS
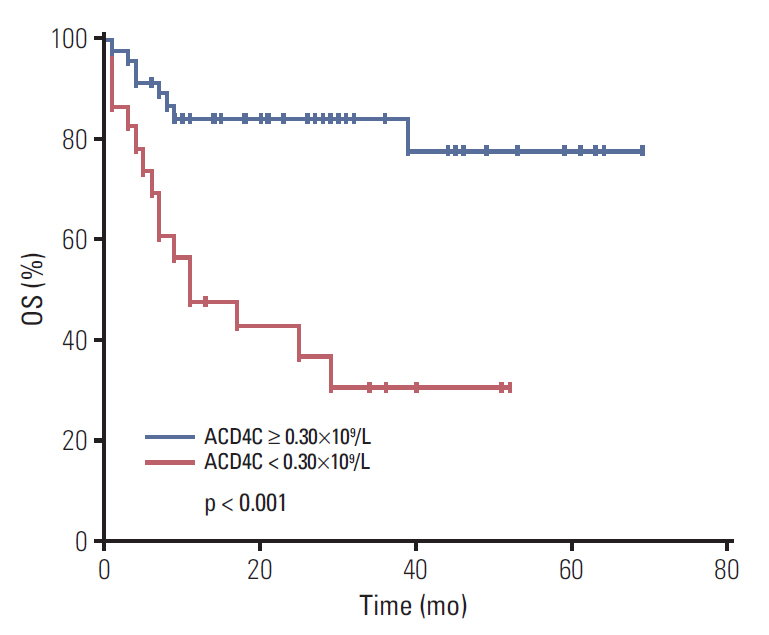
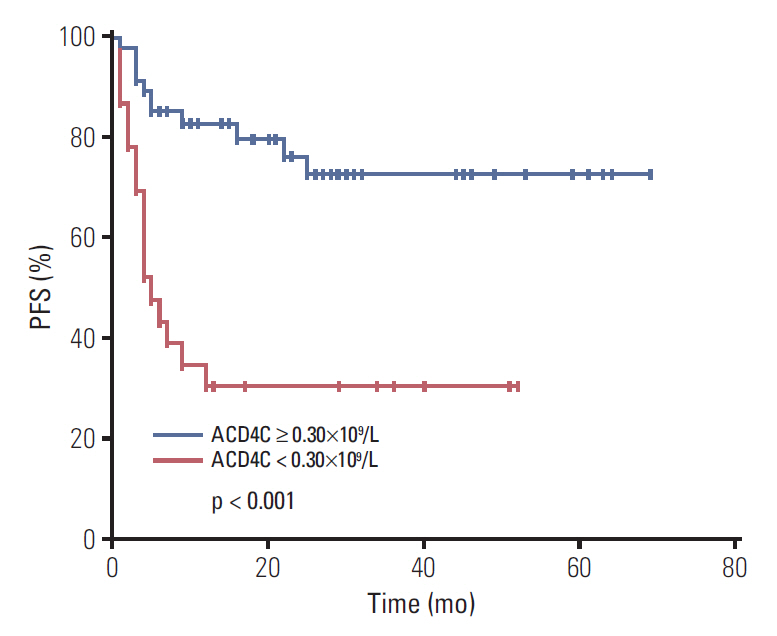
Stage III/IV, B symptoms, elevated lactate dehydrogenase, monocytopenia, high-intermediate and high risk International Prognostic Index (IPI) and Korean Prognostic Index (KPI), high risk Prognostic Index of Natural Killer Lymphoma (PINK), and lower lymphocytes were significantly associated with low ACD4C at diagnosis. With a median follow-up time of 32 months, patients who had an ACD4C < 0.30×109/L had a worse OS. Median OS was 11 months and median PFS was 5 months in the low ACD4C cohort. There were significant differences in both OS and PFS between the two cohorts. Moreover, multivariate Cox analysis identified ACD4Cs as an independent predictor for OS and PFS.
CONCLUSION
Low ACD4Cs were associated with poorer survival and could act as a negative predictor for ENKTL patients treated with asparaginase-based regimens.
Keyword
MeSH Terms
-
Cell Count*
Cohort Studies
Diagnosis
Disease-Free Survival
Drug Therapy*
Follow-Up Studies
Humans
L-Lactate Dehydrogenase
Lymphocyte Subsets
Lymphocytes
Lymphoma*
Lymphoma, Extranodal NK-T-Cell
Lymphoma, Non-Hodgkin
Medical Records
Multivariate Analysis
Prognosis*
Retrospective Studies
T-Lymphocytes
L-Lactate Dehydrogenase
Figure
Reference
-
References
1. Yang QP, Zhang WY, Yu JB, Zhao S, Xu H, Wang WY, et al. Subtype distribution of lymphomas in Southwest China: analysis of 6,382 cases using WHO classification in a single institution. Diagn Pathol. 2011; 6:77.
Article2. Xu W, Fan L, Miao Y, Xu H, Yu L, Xu X, et al. Distribution of lymphomas subtypes in Jiangsu Province: a multicenter analysis of 5 147 cases. Zhonghua Xue Ye Xue Za Zhi. 2014; 35:300–3.3. Au WY, Weisenburger DD, Intragumtornchai T, Nakamura S, Kim WS, Sng I, et al. Clinical differences between nasal and extranasal natural killer/T-cell lymphoma: a study of 136 cases from the International Peripheral T-Cell Lymphoma Project. Blood. 2009; 113:3931–7.
Article4. Perry AM, Diebold J, Nathwani BN, MacLennan KA, Muller-Hermelink HK, Bast M, et al. Non-Hodgkin lymphoma in the Far East: review of 730 cases from the international non-Hodgkin lymphoma classification project. Ann Hematol. 2016; 95:245–51.
Article5. Lee J, Suh C, Park YH, Ko YH, Bang SM, Lee JH, et al. Extranodal natural killer T-cell lymphoma, nasal-type: a prognostic model from a retrospective multicenter study. J Clin Oncol. 2006; 24:612–8.
Article6. Kim SJ, Yoon DH, Jaccard A, Chng WJ, Lim ST, Hong H, et al. A prognostic index for natural killer cell lymphoma after non-anthracycline-based treatment: a multicentre, retrospective analysis. Lancet Oncol. 2016; 17:389–400.
Article7. Peng RJ, Huang ZF, Zhang YL, Yuan ZY, Xia Y, Jiang WQ, et al. Circulating and tumor-infiltrating Foxp3(+) regulatory T cell subset in Chinese patients with extranodal NK/T cell lymphoma. Int J Biol Sci. 2011; 7:1027–36.
Article8. Kwong YL, Chan TS, Tan D, Kim SJ, Poon LM, Mow B, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017; 129:2437–42.
Article9. Huang JJ, Jiang WQ, Lin TY, Huang Y, Xu RH, Huang HQ, et al. Absolute lymphocyte count is a novel prognostic indicator in extranodal natural killer/T-cell lymphoma, nasal type. Ann Oncol. 2011; 22:149–55.
Article10. Wang L, Wang JH, Wu-Xiao ZJ, Xia ZJ, Huang HQ, Lu Y. Lymphopenia during routine follow-up may predict relapse in patients with extranodal NK/T cell lymphoma. Tumour Biol. 2015; 36:1747–53.
Article11. Yang Y, Zhang YJ, Zhu Y, Cao JZ, Yuan ZY, Xu LM, et al. Prognostic nomogram for overall survival in previously untreated patients with extranodal NK/T-cell lymphoma, nasal-type: a multicenter study. Leukemia. 2015; 29:1571–7.
Article12. Suzuki R, Suzumiya J, Yamaguchi M, Nakamura S, Kameoka J, Kojima H, et al. Prognostic factors for mature natural killer (NK) cell neoplasms: aggressive NK cell leukemia and extranodal NK cell lymphoma, nasal type. Ann Oncol. 2010; 21:1032–40.
Article13. He L, Liang JH, Wu JZ, Li Y, Qin SC, Miao Y, et al. Low absolute CD4(+) T cell counts in peripheral blood are associated with inferior survival in follicular lymphoma. Tumour Biol. 2016; 37:12589–95.
Article14. Zhang XY, Xu J, Zhu HY, Wang Y, Wang L, Fan L, et al. Negative prognostic impact of low absolute CD4(+) T cell counts in peripheral blood in mantle cell lymphoma. Cancer Sci. 2016; 107:1471–6.15. Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010; 102:83–7.16. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bioinformatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004; 10:7252–9.17. Gaulard P, de Leval L. The microenvironment in T-cell lymphomas: emerging themes. Semin Cancer Biol. 2014; 24:49–60.
Article18. Coupland SE. The challenge of the microenvironment in B-cell lymphomas. Histopathology. 2011; 58:69–80.
Article19. Blaker YN, Spetalen S, Brodtkorb M, Lingjaerde OC, Beiske K, Ostenstad B, et al. The tumour microenvironment influences survival and time to transformation in follicular lymphoma in the rituximab era. Br J Haematol. 2016; 175:102–14.
Article20. Wein F, Kuppers R. The role of T cells in the microenvironment of Hodgkin lymphoma. J Leukoc Biol. 2016; 99:45–50.
Article21. Chang C, Wu SY, Kang YW, Lin KP, Chen TY, Medeiros LJ, et al. High levels of regulatory T cells in blood are a poor prognostic factor in patients with diffuse large B-cell lymphoma. Am J Clin Pathol. 2015; 144:935–44.
Article22. Chim CS, Ma SY, Au WY, Choy C, Lie AK, Liang R, et al. Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood. 2004; 103:216–21.
Article23. Kim WY, Jeon YK, Kim TM, Kim JE, Kim YA, Lee SH, et al. Increased quantity of tumor-infiltrating FOXP3-positive regulatory T cells is an independent predictor for improved clinical outcome in extranodal NK/T-cell lymphoma. Ann Oncol. 2009; 20:1688–96.
Article24. Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, et al. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009; 27:186–92.
Article25. Hsieh PP, Tung CL, Chan AB, Liao JB, Wang JS, Tseng HH, et al. EBV viral load in tumor tissue is an important prognostic indicator for nasal NK/T-cell lymphoma. Am J Clin Pathol. 2007; 128:579–84.
Article26. Ding ZC, Huang L, Blazar BR, Yagita H, Mellor AL, Munn DH, et al. Polyfunctional CD4(+) T cells are essential for eradicating advanced B-cell lymphoma after chemotherapy. Blood. 2012; 120:2229–39.
Article27. Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010; 70:8368–77.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent updates on extranodal NK/T-cell lymphoma
- Extranodal NK/T cell lymphoma
- A Case of the Nasal Type of Extranodal NK/T-Cell Lymphoma That Mimicked Pyoderma Gangrenosum
- Extranodal NK/T Cell Lymphoma, Nasal Type that Occurred in Patients with Atrophic Rhinitis
- A Case of NK/T Cell Lymphoma in Sinonasal Tract