Ann Rehabil Med.
2018 Oct;42(5):690-701. 10.5535/arm.2018.42.5.690.
A New Functional Scale and Ambulatory Functional Classification of Duchenne Muscular Dystrophy: Scale Development and Preliminary Analyses of Reliability and Validity
- Affiliations
-
- 1Department of Rehabilitation Medicine, Seoul National University College of Medicine, Seoul, Korea. msbang@snu.ac.kr
- 2Ewha Brain Institute, Ewha Womans University, Seoul, Korea.
- 3Department of Physical Medicine and Rehabilitation, Chungnam National University Hospital, Daejeon, Korea.
- 4Department of Physical and Rehabilitation Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Medical Research Collaborating Center, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 6Department of Preventive Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 7Department of Biomedical Science, Graduate School of Seoul National University, Seoul, Korea.
- 8Cancer Research Institute, Seoul National University, Seoul, Korea.
- KMID: 2429198
- DOI: http://doi.org/10.5535/arm.2018.42.5.690
Abstract
OBJECTIVE
To develop a simplified functional scale and classification system to evaluate the functional abilities of patients with Duchenne muscular dystrophy (DMD).
METHODS
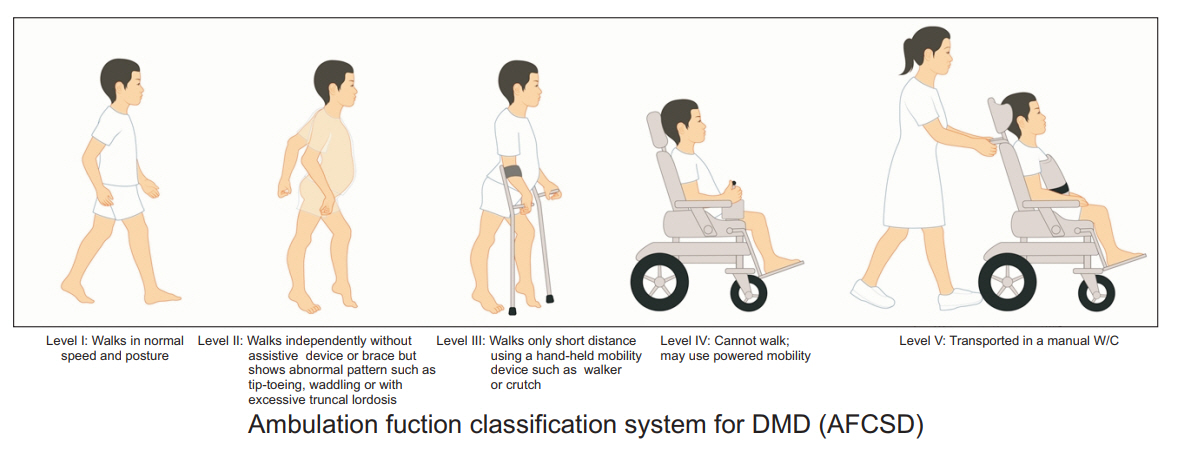
A Comprehensive Functional Scale for DMD (CFSD) was developed using the modified Delphi method. The accompanying Ambulatory Functional Classification System for DMD (AFCSD) was developed based on previously published classification systems.
RESULTS
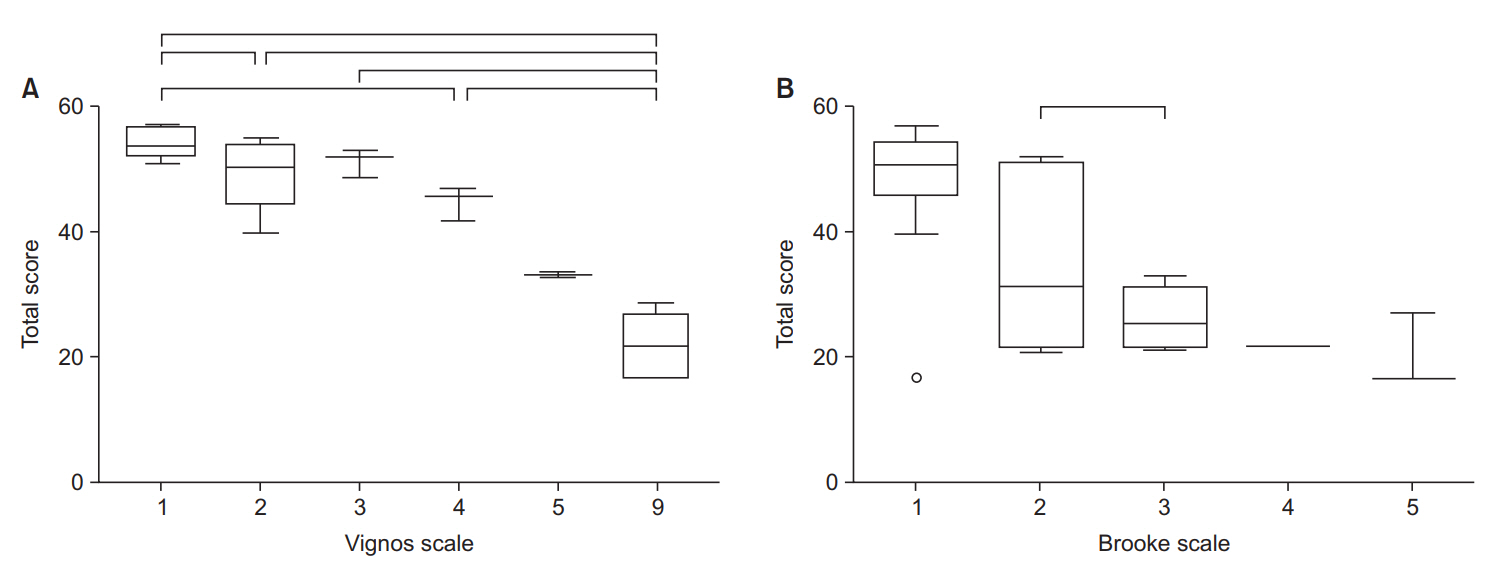
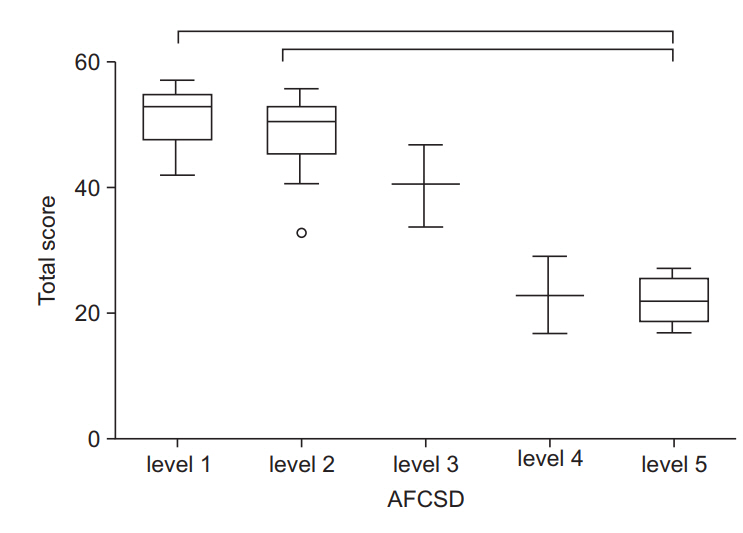
The CFSD consists of 21 items and 78 sub-items, assessing body structure and function, activities, and participation. Inter-rater intraclass correlation coefficient values were above 0.7 for 17 items. The overall limits of agreement between the two examiners ranged from -6.21 to 3.11. The Spearman correlation coefficient between the total score on the AFCSD and the Vignos Functional Scale was 0.833, and 0.714 between the total score of the AFCSD and the Brooke scale. Significant negative correlations existed between the total score for each functional level of the AFCSD and each functional grade of the Vignos and Brooke scales. The total scores of the CFSD varied significantly between the functional grades of the Vignos scale, and specific grades of the Brooke scale. For the AFCSD, total scores of the CFSD varied significantly between the functional levels.
CONCLUSION
We have developed a new scale and the associated classification system, to assess the functional ability of children diagnosed with DMD. Preliminary evaluation of the psychometric properties of the functional scale and classification systems indicate sufficient reliability and concurrent validity.
MeSH Terms
Figure
Cited by 2 articles
-
Reliability and Validity of the Korean Version of the Duchenne Muscular Dystrophy Functional Ability Self-Assessment Tool
Kyunghyun Lee, Sung Eun Hyun, Hyung-Ik Shin, Hye Min Ji
Ann Rehabil Med. 2023;47(2):79-88. doi: 10.5535/arm.23013.Muscle Pathology Associated With Cardiac Function in Duchenne Muscular Dystrophy
Jin A Yoon, Heirim Lee, In Sook Lee, You Seon Song, Byeong-Ju Lee, Soo-Yeon Kim, Yong Beom Shin
Ann Rehabil Med. 2024;48(6):405-412. doi: 10.5535/arm.240006.
Reference
-
1. Moxley RT 3rd, Pandya S, Ciafaloni E, Fox DJ, Campbell K. Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J Child Neurol. 2010; 25:1116–29.
Article2. Voit T, Topaloglu H, Straub V, Muntoni F, Deconinck N, Campion G, et al. Safety and efficacy of drisapersen for the treatment of Duchenne muscular dystrophy (DEMAND II): an exploratory, randomised, placebocontrolled phase 2 study. Lancet Neurol. 2014; 13:987–96.
Article3. Seto JT, Bengtsson NE, Chamberlain JS. Therapy of genetic disorders-novel therapies for Duchenne muscular dystrophy. Curr Pediatr Rep. 2014; 2:102–12.4. Bakker JP, de Groot IJ, Beckerman H, de Jong BA, Lankhorst GJ. The effects of knee-ankle-foot orthoses in the treatment of Duchenne muscular dystrophy: review of the literature. Clin Rehabil. 2000; 14:343–59.
Article5. Skalsky AJ, McDonald CM. Prevention and management of limb contractures in neuromuscular diseases. Phys Med Rehabil Clin N Am. 2012; 23:675–87.
Article6. Sethi PK. The Knud Jansen lecture. Technological choices in prosthetics and orthotics for developing countries. Prosthet Orthot Int. 1989; 13:117–24.7. Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, et al. Diagnosis and management of Duchenne muscular dystrophy. Part 2. Implementation of multidisciplinary care. Lancet Neurol. 2010; 9:177–89.
Article8. Loos GP, Smith RG, Roseman C. Probable future funding priorities in maternal and child health: a modified Delphi National Survey. J Health Polit Policy Law. 1985; 9:683–93.
Article9. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997; 39:214–23.
Article10. Hidecker MJ, Paneth N, Rosenbaum PL, Kent RD, Lillie J, Eulenberg JB, et al. Developing and validating the Communication Function Classification System for individuals with cerebral palsy. Dev Med Child Neurol. 2011; 53:704–10.
Article11. Eliasson AC, Krumlinde-Sundholm L, Rosblad B, Beckung E, Arner M, Ohrvall AM, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. 2006; 48:549–54.
Article12. Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci. 1999; 169:13–21.13. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J. 1965; 14:61–5.14. Hocking C, Williams M, Broad J, Baskett J. Sensitivity of Shah, Vanclay and Cooper’s modified Barthel Index. Clin Rehabil. 1999; 13:141–7.
Article15. Lue YJ, Su CY, Yang RC, Su WL, Lu YM, Lin RF, et al. Development and validation of a muscular dystrophy-specific functional rating scale. Clin Rehabil. 2006; 20:804–17.
Article16. Krosschell KJ, Maczulski JA, Crawford TO, Scott C, Swoboda KJ. A modified Hammersmith functional motor scale for use in multi-center research on spinal muscular atrophy. Neuromuscul Disord. 2006; 16:417–26.
Article17. van den Beld WA, van der Sanden GA, Feuth T, Janssen AJ, Sengers RC, Verbeek AL, et al. A new motor performance test in a prospective study on children with suspected myopathy. Dev Med Child Neurol. 2006; 48:739–43.
Article18. Ostensjo S, Bjorbaekmo W, Carlberg EB, Vollestad NK. Assessment of everyday functioning in young children with disabilities: an ICF-based analysis of concepts and content of the Pediatric Evaluation of Disability Inventory (PEDI). Disabil Rehabil. 2006; 28:489–504.19. Brooke MH, Griggs RC, Mendell JR, Fenichel GM, Shumate JB, Pellegrino RJ. Clinical trial in Duchenne dystrophy. I. The design of the protocol. Muscle Nerve. 1981; 4:186–97.
Article20. Ottenbacher KJ, Taylor ET, Msall ME, Braun S, Lane SJ, Granger CV, et al. The stability and equivalence reliability of the functional independence measure for children (WeeFIM). Dev Med Child Neurol. 1996; 38:907–16.
Article21. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969; 9:179–86.
Article22. Jung IY, Chae JH, Park SK, Kim JH, Kim JY, Kim SJ, et al. The correlation analysis of functional factors and age with Duchenne muscular dystrophy. Ann Rehabil Med. 2012; 36:22–32.
Article23. Ricotti V, Ridout DA, Pane M, Main M, Mayhew A, Mercuri E, et al. The NorthStar Ambulatory Assessment in Duchenne muscular dystrophy: considerations for the design of clinical trials. J Neurol Neurosurg Psychiatry. 2016; 87:149–55.
Article24. Kinali M, Messina S, Mercuri E, Lehovsky J, Edge G, Manzur AY, et al. Management of scoliosis in Duchenne muscular dystrophy: a large 10-year retrospective study. Dev Med Child Neurol. 2006; 48:513–8.
Article25. Kinali M, Main M, Eliahoo J, Messina S, Knight RK, Lehovsky J, et al. Predictive factors for the development of scoliosis in Duchenne muscular dystrophy. Eur J Paediatr Neurol. 2007; 11:160–6.
Article26. Mullender M, Blom N, De Kleuver M, Fock J, Hitters W, Horemans A, et al. A Dutch guideline for the treatment of scoliosis in neuromuscular disorders. Scoliosis. 2008; 3:14.
Article27. Weis R. Introduction to abnormal child and adolescent psychology. 2nd ed. Los Angeles: Sage Publications;2014.28. AAIDD User’s Guide Work Group. User’s guide: to accompany the 11th edition of Intellectual disability: definition, classification, and systems of supports: applications for clinicians, educators, organizations providing supports, policymakers, family members and advocates, and health care professionals. Washington: American Association on Intellectual and Developmental Disabilities;2012.29. Bittles AH, Petterson BA, Sullivan SG, Hussain R, Glasson EJ, Montgomery PD. The influence of intellectual disability on life expectancy. J Gerontol A Biol Sci Med Sci. 2002; 57:M470–2.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reliability and Validity of the Korean Version of the Duchenne Muscular Dystrophy Functional Ability Self-Assessment Tool
- Clinical Implications of Pulmonary Function Test and Maximum Static Pressure in Duchenne Muscular Dystrophy
- Acute Gastric Dilatation and Calculous Cholecystitis in Duchenne's Muscular Dystrophy
- A clinical study on Duchenne muscular dystrophy
- Correction: Reliability and Validity of the Korean Version of the Duchenne Muscular Dystrophy Functional Ability Self-Assessment Tool