Yonsei Med J.
2019 Jan;60(1):98-105. 10.3349/ymj.2019.60.1.98.
The Usefulness of Muscle Biopsy in Initial Diagnostic Evaluation of Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes
- Affiliations
-
- 1Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea. ymleemd@yuhs.ac
- 2Department of Pathology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2428283
- DOI: http://doi.org/10.3349/ymj.2019.60.1.98
Abstract
- PURPOSE
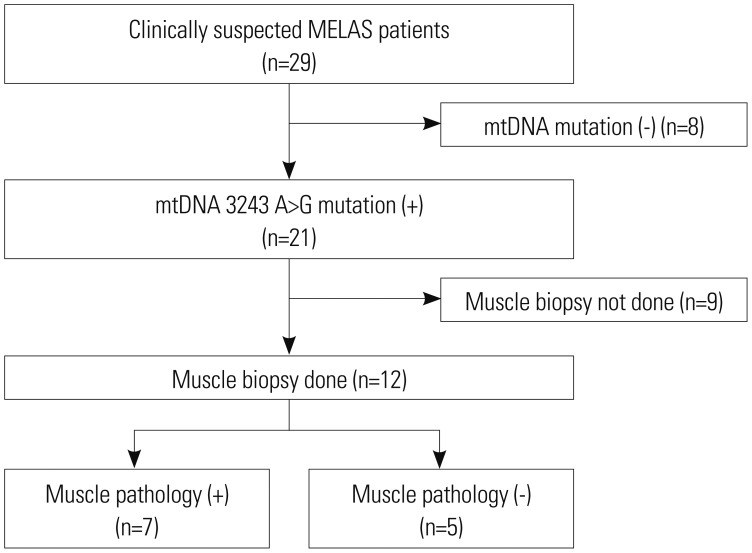
The disease entity mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) is characterized by an early onset of stroke-like episodes. MELAS is the most dominant subtype of mitochondrial disease. Molecular genetic testing is important in the diagnosis of MELAS. The mitochondrial DNA (mtDNA) 3243A>G mutation is found in 80% of MELAS patients. Nevertheless, molecular analysis alone may be insufficient to diagnose MELAS because of mtDNA heteroplasmy. This study aimed to evaluate whether muscle biopsy is useful in MELAS patients as an initial diagnostic evaluation method.
MATERIALS AND METHODS
The medical records of patients who were diagnosed with MELAS at the Department of Pediatrics of Gangnam Severance Hospital between January 2006 and January 2017 were reviewed. The study population included 12 patients. They were divided into two subgroups according to whether the results of muscle pathology were in accordance with mitochondrial diseases. Clinical variables, diagnostic evaluations, and clinical outcomes were compared between the two groups.
RESULTS
Of the 12 patients, seven were muscle pathology-positive for mitochondrial disease. No statistically significant difference in clinical data was observed between the groups that were muscle pathology-positive and muscle pathology-negative for mtDNA 3243A>G mutation. Additionally, the patients with weakness as the initial symptom were all muscle pathology-positive.
CONCLUSION
The usefulness of muscle biopsy appears to be limited to an initial confirmative diagnostic evaluation of MELAS. Muscle biopsy can provide some information in MELAS patients with weakness not confirmed by genetic testing.
MeSH Terms
Figure
Cited by 2 articles
-
The Author Reply: Mitochondrial Ophthalmoplegia Is Not Only due to mtDNA Deletions
Young-Mock Lee
Yonsei Med J. 2019;60(2):232-233. doi: 10.3349/ymj.2019.60.2.232.Genetic Data Are a Prerequisite for Interpreting Clinical and Muscle Biopsy Findings in MELAS
Josef Finsterer
Yonsei Med J. 2019;60(4):399-400. doi: 10.3349/ymj.2019.60.4.399.
Reference
-
1. Zeviani M, Di Donato S. Mitochondrial disorders. Brain. 2004; 127(Pt 10):2153–2172. PMID: 15358637.
Article2. Yatsuga S, Povalko N, Nishioka J, Katayama K, Kakimoto N, Matsuishi T, et al. MELAS: a nationwide prospective cohort study of 96 patients in Japan. Biochim Biophys Acta. 2012; 1820:619–624. PMID: 21443929.
Article3. Goto Y, Horai S, Matsuoka T, Koga Y, Nihei K, Kobayashi M, et al. Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS): a correlative study of the clinical features and mitochondrial DNA mutation. Neurology. 1992; 42(3 Pt 1):545–550. PMID: 1549215.4. Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984; 16:481–488. PMID: 6093682.
Article5. Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990; 348:651–653. PMID: 2102678.
Article6. El-Hattab AW, Adesina AM, Jones J, Scaglia F. MELAS syndrome: clinical manifestations, pathogenesis, and treatment options. Mol Genet Metab. 2015; 116:4–12. PMID: 26095523.
Article7. Kisler JE, Whittaker RG, McFarland R. Mitochondrial diseases in childhood: a clinical approach to investigation and management. Dev Med Child Neurol. 2010; 52:422–433. PMID: 20163433.
Article8. Haas RH, Parikh S, Falk MJ, Saneto RP, Wolf NI, Darin N, et al. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007; 120:1326–1333. PMID: 18055683.
Article9. Koenig MK. Presentation and diagnosis of mitochondrial disorders in children. Pediatr Neurol. 2008; 38:305–313. PMID: 18410845.
Article10. Parikh S, Goldstein A, Koenig MK, Scaglia F, Enns GM, Saneto R, et al. Diagnosis and management of mitochondrial disease: a consensus statement from the Mitochondrial Medicine Society. Genet Med. 2015; 17:689–701. PMID: 25503498.
Article11. Hirano M, Ricci E, Koenigsberger MR, Defendini R, Pavlakis SG, DeVivo DC, et al. Melas: an original case and clinical criteria for diagnosis. Neuromuscul Disord. 1992; 2:125–135. PMID: 1422200.
Article12. Parsons T, Weimer L, Engelstad K, Linker A, Battista V, Wei Y, et al. Autonomic symptoms in carriers of the m.3243A>G mitochondrial DNA mutation. Arch Neurol. 2010; 67:976–979. PMID: 20697048.
Article13. Morten KJ, Cooper JM, Brown GK, Lake BD, Pike D, Poulton J. A new point mutation associated with mitochondrial encephalomyopathy. Hum Mol Genet. 1993; 2:2081–2087. PMID: 8111377.
Article14. Moraes CT, Ciacci F, Silvestri G, Shanske S, Sciacco M, Hirano M, et al. Atypical clinical presentations associated with the MELAS mutation at position 3243 of human mitochondrial DNA. Neuromuscul Disord. 1993; 3:43–50. PMID: 8392410.
Article15. Nesbitt V, Pitceathly RD, Turnbull DM, Taylor RW, Sweeney MG, Mudanohwo EE, et al. The UK MRC Mitochondrial Disease Patient Cohort Study: clinical phenotypes associated with the m.3243A>G mutation--implications for diagnosis and management. J Neurol Neurosurg Psychiatry. 2013; 84:936–938. PMID: 23355809.16. Uusimaa J, Moilanen JS, Vainionpää L, Tapanainen P, Lindholm P, Nuutinen M, et al. Prevalence, segregation, and phenotype of the mitochondrial DNA 3243A>G mutation in children. Ann Neurol. 2007; 62:278–287. PMID: 17823937.17. Manwaring N, Jones MM, Wang JJ, Rochtchina E, Howard C, Mitchell P, et al. Population prevalence of the MELAS A3243G mutation. Mitochondrion. 2007; 7:230–233. PMID: 17300999.
Article18. Sproule DM, Kaufmann P. Mitochondrial encephalopathy, lactic acidosis, and strokelike episodes: basic concepts, clinical phenotype, and therapeutic management of MELAS syndrome. Ann N Y Acad Sci. 2008; 1142:133–158. PMID: 18990125.19. Rollins S, Prayson RA, McMahon JT, Cohen BH. Diagnostic yield muscle biopsy in patients with clinical evidence of mitochondrial cytopathy. Am J Clin Pathol. 2001; 116:326–330. PMID: 11554158.20. Goto Y. Clinical features of MELAS and mitochondrial DNA mutations. Muscle Nerve Suppl. 1995; 3:S107–S112. PMID: 7603510.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Status Epilepticus as the Initial Manifestation of Mitochondrial Myopathy, Encephalopathy, Lactic Acidosis, and Stroke-Like Episodes Syndrome
- A Two Cases of MELAS in Siblings
- Atypical Radiologic Manifestation of NARP Mimicking MELAS: a Case Report
- A Case of MELAS Syndrome Diagnosed in a Woman in Her 50s
- Clinical Value of Magnetic Resonance Spectroscopy in the Initial Evaluation of Patients with Mitochondrial Encephalomyopathy, Lactic Acidosis, and Stroke-Like Episodes