Clin Endosc.
2018 Sep;51(5):463-469. 10.5946/ce.2018.012.
Experimental Study to Develop a Method for Improving Sample Collection to Monitor Laryngoscopes after Reprocessing
- Affiliations
-
- 1Department of Public Health and Pediatrics, University of Turin, Turin, Italy. savina.ditommaso@unito.it
- KMID: 2427719
- DOI: http://doi.org/10.5946/ce.2018.012
Abstract
- BACKGROUND/AIMS
The microbiological surveillance of endoscopes and automated flexible endoscope reprocessing have been proven to be two of the most difficult and controversial areas of infection control in endoscopy. The purpose of this study was to standardize a sampling method for assessing the effectiveness of standard reprocessing operating procedures for flexible fiberoptic laryngoscopes (FFLs).
METHODS
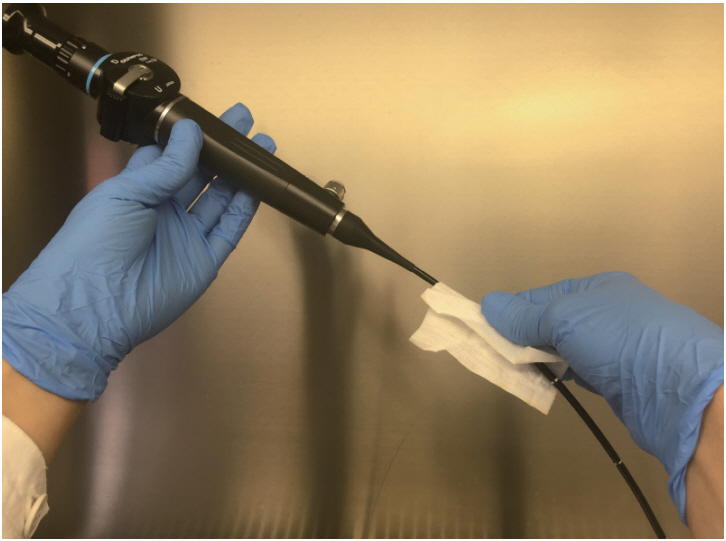
First, the sampling devices were directly inoculated with Bacillus atrophaeus spores; second, tissue non tissue (TNT) wipes were tested on artificially contaminated surfaces and on FFLs.
RESULTS
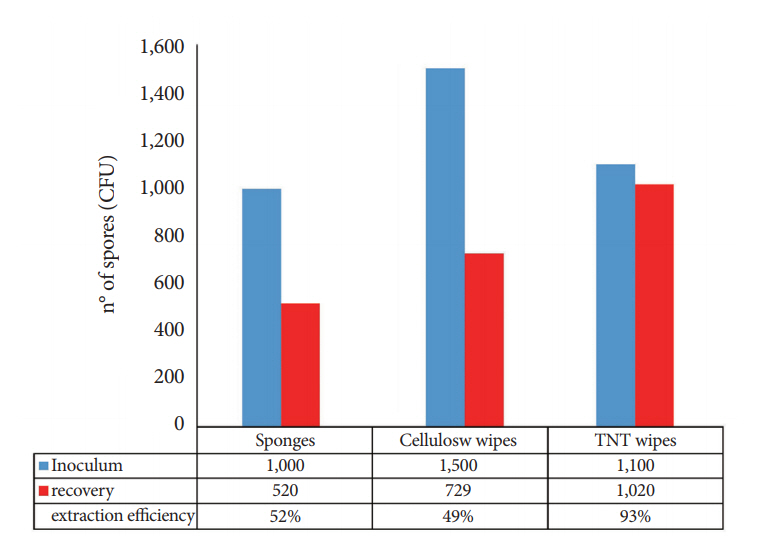
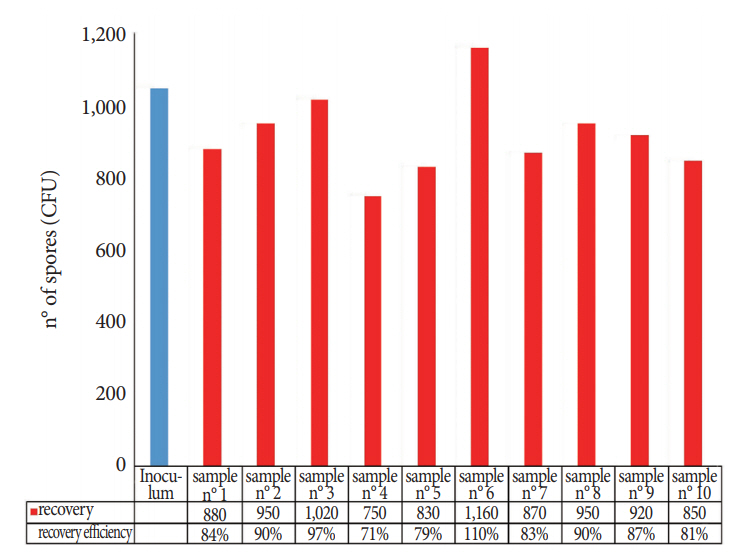
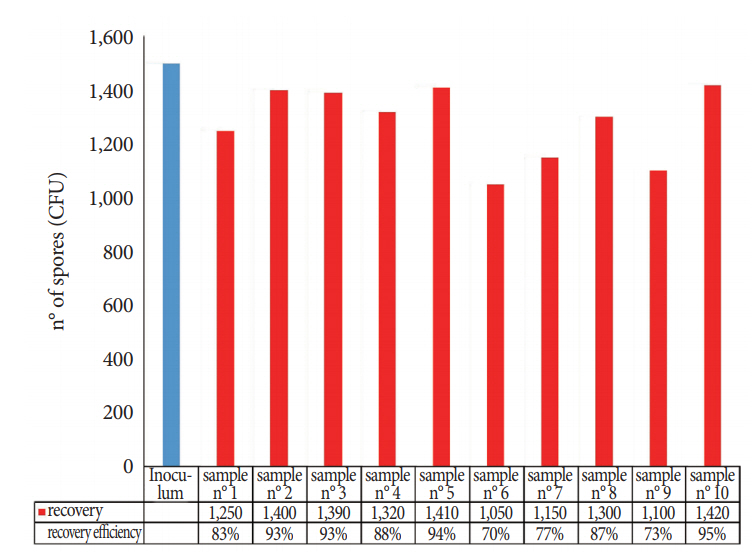
Comparison of the sponges, cellulose, and TNT wipes indicated that the TNT wipes were more effective in releasing spores (93%) than the sponges (49%) and cellulose wipes (52%). The developed protocol provides a high efficiency for both collection and extraction from the stainless steel surface (87% of the spores were removed and released) and from the FFL (85% of the spores were removed and released), with relatively low standard deviations for recovery efficiency, particularly for the analysis of the FFL.
CONCLUSIONS
TNT wipes are more efficient for sampling surface areas, thereby aiding in the accuracy and reproducibility of environmental surveillance.
MeSH Terms
Figure
Cited by 1 articles
-
What is the Best Sampling Method to Monitor the Effect of Endoscopy Reprocessing?
Kwang Hyun Chung, Byung Ik Jang
Clin Endosc. 2018;51(5):397-398. doi: 10.5946/ce.2018.146.
Reference
-
1. Sehulster LM, Chinn RYW, Arduino MJ, et al. Guidelines for environmental infection control in health-care facilities. Recommendations from CDC and the Healthcare Infection Control Practices Advisory Committee (HICPAC). Chicago (IL): American Society for Healthcare Engineering/American Hospital Association;2004.2. Edmonds JM. Efficient methods for large-area surface sampling of sites contaminated with pathogenic microorganisms and other hazardous agents: current state, needs, and perspectives. Appl Microbiol Biotechnol. 2009; 84:811–816.
Article3. Buttner MP, Cruz P, Stetzenbach LD, Cronin T. Evaluation of two surface sampling methods for detection of Erwinia herbicola on a variety of materials by culture and quantitative PCR. Appl Environ Microbiol. 2007; 73:3505–3510.4. Edmonds JM, Collett PJ, Valdes ER, Skowronski EW, Pellar GJ, Emanuel PA. Surface sampling of spores in dry-deposition aerosols. Appl Environ Microbiol. 2009; 75:39–44.
Article5. Moore G, Griffith C. A comparison of traditional and recently developed methods for monitoring surface hygiene within the food industry: an industry trial. Int J Environ Health Res. 2002; 12:317–329.
Article6. Sanderson WT, Hein MJ, Taylor L, et al. Surface sampling methods for Bacillus anthracis spore contamination. Emerg Infect Dis. 2002; 8:1145–1151.7. Vorst KL, Todd EC, Rysert ET. Improved quantitative recovery of Listeria monocytogenes from stainless steel surfaces using a one-ply composite tissue. J Food Prot. 2004; 67:2212–2217.
Article8. Brown GS, Betty RG, Brockmann JE, et al. Evaluation of rayon swab surface sample collection method for Bacillus spores from nonporous surfaces. J Appl Microbiol. 2007; 103:1074–1080.9. Downey AS, Da Silva SM, Olson ND, Filliben JJ, Morrow JB. Impact of processing method on recovery of bacteria from wipes used in biological surface sampling. Appl Environ Microbiol. 2012; 78:5872–5881.
Article10. Ruple-Czerniak A, Bolte DS, Burgess BA, Morley PS. Comparison of two sampling and culture systems for detection of Salmonella enterica in the environment of a large animal hospital. Equine Vet J. 2014; 46:499–502.11. Bredholt S, Maukonen J, Kujanpää K, et al. Microbial methods for assessment of cleaning and disinfection of food-processing surfaces cleaned in a low-pressure system. Eur Food Res Technol. 1999; 209:145–152.
Article12. Digestive Health Foundation. Infection control in endoscopy [Internet]. Sydney: Gastroenterological Society of Australia;c2008. [updated 2008 Feb; cited 2018 Jul 11]. Available from: http://cart.gesa.org.au/membes/files/Clinical%20Guidelines%20and%20Updates/Endoscopy_Microbiological_Testing.pdf.13. Centers for Disease Control and Prevention. Duodenoscope surveillance sampling & culturing: reducing the risks of infection [Internet]. Atlanta (GA): CDC;c2015. [updated 2008 Feb; cited 2018 Jul 11]. Available from: https://www.cdc.gov/hai/organisms/cre/cre-duodenoscope-surveillance-protocol.html.14. Ministère de la santé et des solidarités. Elements d’assurance qualite en hygiene relatifs au contrôle microbiologique des endoscopes et à la traçabilite en endoscopie [Internet]. Paris: Société Française d’Endoscopie Digestive;c2007. [cited 2018 Jul 11]. Available from: http://www.sfed.org/professionnels/commissions-et-actions/plateaux-techniques-qualite-hygiene-et-securite/qualite/textes.15. Bhatt JM, Peterson EM, Verma SP. Microbiological sampling of the forgotten components of a flexible fiberoptic laryngoscope: what lessons can we learn? Otolaryngol Head Neck Surg. 2014; 150:235–236.16. Elackattu A, Zoccoli M, Spiegel JH, Grundfast KM. A comparison of two methods for preventing cross-contamination when using flexible fiberoptic endoscopes in an otolaryngology clinic: disposable sterile sheaths versus immersion in germicidal liquid. Laryngoscope. 2010; 120:2410–2416.
Article17. Tzanidakis K, Choudhury N, Bhat S, Weerasinghe A, Marais J. Evaluation of disinfection of flexible nasendoscopes using Tristel wipes: a prospective single blind study. Ann R Coll Surg Engl. 2012; 94:185–188.
Article18. Bhattacharyya N, Kepnes LJ. The effectiveness of immersion disinfection for flexible fiberoptic laryngoscopes. Otolaryngol Head Neck Surg. 2004; 130:681–685.
Article19. Liming B, Funnell I, Jones A, Demons S, Marshall K, Harsha W. An evaluation of varying protocols for high-level disinfection of flexible fiberoptic laryngoscopes. Laryngoscope. 2014; 124:2498–2501.
Article20. International Organization for Standardization. ISO 11737-1:2006. Sterilization of medical devices -- microbiological methods -- part 1: determination of a population of microorganisms on products [Internet]. Geneva: ISO;c2006. [updated 2006 Apr; cited 2018 Jul 11]. Available from: https://www.iso.org/standard/38711.html.21. European Committee for Standardization. EN 14347 - Chemical disinfectants and antiseptics - basic sporicidal activity - test method and requirements (phase 1, step 1). Brussels: European Standards;2005.22. European Committee for Standardization. EN 13704 - Chemical disinfectants - quantitative suspension test for evaluation of sporicidal activity of chemical disinfectants used in food, industrial, domestic and institutional areas - test method and requirements (phase 2, step 1). Brussels: European Standards;2002.23. International Organization for Standardization. ISO/TR 13843: Water quality - guidance on validation of microbiological methods [Internet]. Geneva: ISO;c2000. [updated 2000 Jun; cited 2018 Jul 11]. Available from: https://www.iso.org/standard/22599.html.24. ASGE Quality Assurance In Endoscopy Committee, Petersen BT, Chennat J, et al. Multisociety guideline on reprocessing flexible gastrointestinal endoscopes: 2011. Gastrointest Endosc. 2011; 73:1075–1084.
Article25. Lee YK, Park JB. Steps of reprocessing and equipments. Clin Endosc. 2013; 46:274–279.
Article26. Beilenhoff U, Neumann CS, Rey JF, Biering H, Blum R, Schmidt V. ESGE-ESGENA guideline for quality assurance in reprocessing: microbiological surveillance testing in endoscopy. Endoscopy. 2007; 39:175–181.
Article27. Saviuc P, Picot-Guéraud R, Shum Cheong Sing J, et al. Evaluation of the quality of reprocessing of gastrointestinal endoscopes. Infect Control Hosp Epidemiol. 2015; 36:1017–1023.
Article28. Epstein L, Hunter JC, Arwady MA, et al. New Delhi metallo-beta-lactamase-producing carbapenem-resistant Escherichia coli associated with exposure to duodenoscopes. JAMA. 2014; 312:1447–1455.29. Muscarella LF. Prevention of disease transmission during flexible laryngoscopy. Am J Infect Control. 2007; 35:536–544.
Article30. Spaulding EH. Chemical disinfection and antisepsis in the hospital. J Hosp Res. 1972; 9:5–31.31. Collins WO. A review of reprocessing techniques of flexible nasopharyngoscopes. Otolaryngol Head Neck Surg. 2009; 141:307–310.
Article32. McDonnell G, Burke P. Disinfection: is it time to reconsider Spaulding? J Hosp Infect. 2011; 78:163–170.
Article33. Lutz JK, Crawford J, Hoet AE, Wilkins JR 3rd, Lee J. Comparative performance of contact plates, electrostatic wipes, swabs and a novel sampling device for the detection of Staphylococcus aureus on environmental surfaces. J Appl Microbiol. 2013; 115:171–178.
Article34. Rose LJ, Hodges L, O’Connell H, Noble-Wang J. National validation study of a cellulose sponge wipe-processing method for use after sampling Bacillus anthracis spores from surfaces. Appl Environ Microbiol. 2011; 77:8355–8359.
Article