Ann Hepatobiliary Pancreat Surg.
2018 Nov;22(4):412-415. 10.14701/ahbps.2018.22.4.412.
New two-step wedge liver resection technique: “zoom resectionâ€: A case report
- Affiliations
-
- 1Hepatobiliary and Pancreatic Surgical Unit, Sanatorio Güemes-University Hospital, Cuidad Autónoma de Buenos Aires, Argentine. guillermopfaffen@gmail.com
- KMID: 2427374
- DOI: http://doi.org/10.14701/ahbps.2018.22.4.412
Abstract
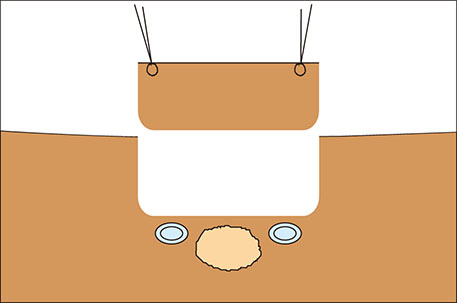
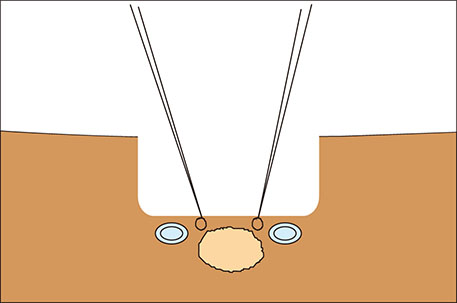
- Different surgical procedures have been described for the treatment of colorectal liver metastases. The appropriate surgical approach depends, among many other factors, on the relationship between liver metastases and suprahepatic veins. If possible, the detachment of colorectal liver metastasis from suprahepatic veins is a good alternative liver parenchyma sparing technique. In this study, we describe a new two-step wedge liver resection technique for colorectal liver metastases located between suprahepatic veins. Prior to resection, intraoperative ultrasound is employed in order to plan and guide both steps. Initially, we place stitches and resect a cylindrical piece of normal liver parenchyma above the tumor and suprahepatic veins. Next, we place stitches on the future specimen located between suprahepatic veins, then resect it. The main advantages of this procedure are the good visualization and vascular control that may be achieved during the detachment of the tumor from suprahepatic veins. We call this procedure "zoom resection" because its dynamics are similar to the workings of a photograph camera's telescopic system. We present the case of a 55-year-old patient diagnosed with multiple colorectal liver metastases, one of which was resected through this technique.
Keyword
Figure
Reference
-
1. Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999; 230:309–318.2. Urbani L, Colombatto P, Balestri R, Licitra G, Leoni C, Forfori F, et al. Techniques of parenchyma-sparing hepatectomy for the treatment of tumors involving the hepatocaval confluence: a reliable way to assure an adequate future liver remnant volume. Surgery. 2017; 162:483–499.3. Donadon M, Torzilli G. From mesohepatectomy to mini-mesohepatectomy: evolving the concept of resectability of hepatic tumors at the hepatocaval confluence. Dig Surg. 2011; 28:109–113.
Article4. de Santibañes E, Sánchez Clariá R, Palavecino M, Beskow A, Pekolj J. Liver metastasis resection: a simple technique that makes it easier. J Gastrointest Surg. 2007; 11:1183–1187.
Article5. van Dam RM, Lodewick TM, van den Broek MA, de Jong MC, Greve JW, Jansen RL, et al. Outcomes of extended versus limited indications for patients undergoing a liver resection for colorectal cancer liver metastases. HPB (Oxford). 2014; 16:550–559.
Article6. Aoki T, Sugawara Y, Imamura H, Seyama Y, Minagawa M, Hasegawa K, et al. Hepatic resection with reconstruction of the inferior vena cava or hepatic venous confluence for metastatic liver tumor from colorectal cancer. J Am Coll Surg. 2004; 198:366–372.7. Margonis GA, Sergentanis TN, Ntanasis-Stathopoulos I, Andreatos N, Tzanninis IG, Sasaki K, et al. Impact of surgical margin width on recurrence and overall survival following R0 hepatic resection of colorectal metastases: a systematic review and meta-analysis. Ann Surg. 2018; 267:1047–1055.8. Torzilli G, Montorsi M, Donadon M, Palmisano A, Del Fabbro D, Gambetti A, et al. “Radical but conservative” is the main goal for ultrasonography-guided liver resection: prospective validation of this approach. J Am Coll Surg. 2005; 201:517–528.
Article9. de Haas RJ, Wicherts DA, Flores E, Azoulay D, Castaing D, Adam R. R1 resection by necessity for colorectal liver metastases: is it still a contraindication to surgery? Ann Surg. 2008; 248:626–637.10. Viganò L, Procopio F, Cimino MM, Donadon M, Gatti A, Costa G, et al. Is tumor detachment from vascular structures equivalent to R0 resection in surgery for colorectal liver metastases? An observational cohort. Ann Surg Oncol. 2016; 23:1352–1360.
Article11. Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005; 241:715–722.
Article12. Dipasco PJ, Misra S, Koniaris LG. Conformational technique for non-anatomic resection of liver lesions. J Gastrointest Surg. 2012; 16:1972–1975.
Article13. Horton PJ, Chaudhury PK, Znajda TL, Martinie JB, Rochon C, Tzimas GN, et al. Novel two-step resection for lesions between the middle hepatic vein and vena cava which allows the middle hepatic vein to be preserved. J Gastrointest Surg. 2006; 10:69–76.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resection of Colorectal Liver Metastases
- Dosimetric Characteristics of Dynamic Wedge Techinique
- LumbarWedgeResectionOsteotomyforCongenitalScoliosis duetoaSacralMalformation: A CaseReport
- How to minimize conversion to open surgery during laparoscopic liver resection: the point of view of hemostasis
- Case series of keloid wedge resection in the ear: a focus on aesthetic aspects