Yonsei Med J.
2016 Nov;57(6):1511-1516. 10.3349/ymj.2016.57.6.1511.
Immunogenicity of MenACWY-CRM in Korean Military Recruits: Influence of Tetanus-Diphtheria Toxoid Vaccination on the Vaccine Response to MenACWY-CRM
- Affiliations
-
- 1Department of Pediatrics, Ewha Womans University School of Medicine, Seoul, Korea. kaykim@ewha.ac.kr
- 2Center for Vaccine Evaluation and Study, Ewha Womans University School of Medicine, Seoul, Korea.
- 3Laboratory of Immunology and Infectious Diseases, Graduate School of Medical Science and Engineering, KAIST, Daejeon, Korea. ecshin@kaist.ac.kr
- KMID: 2427173
- DOI: http://doi.org/10.3349/ymj.2016.57.6.1511
Abstract
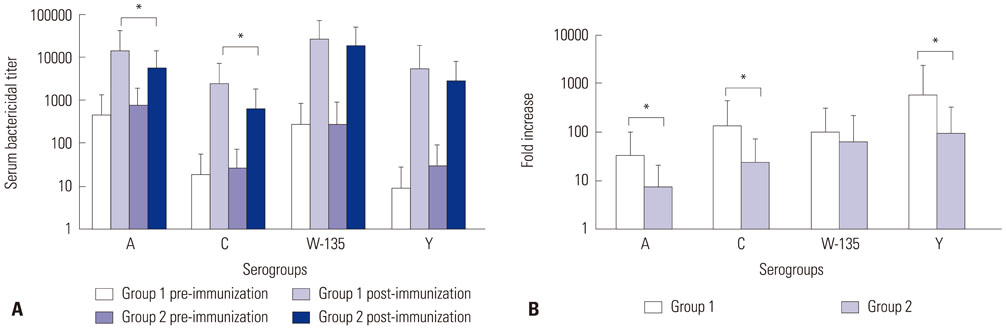
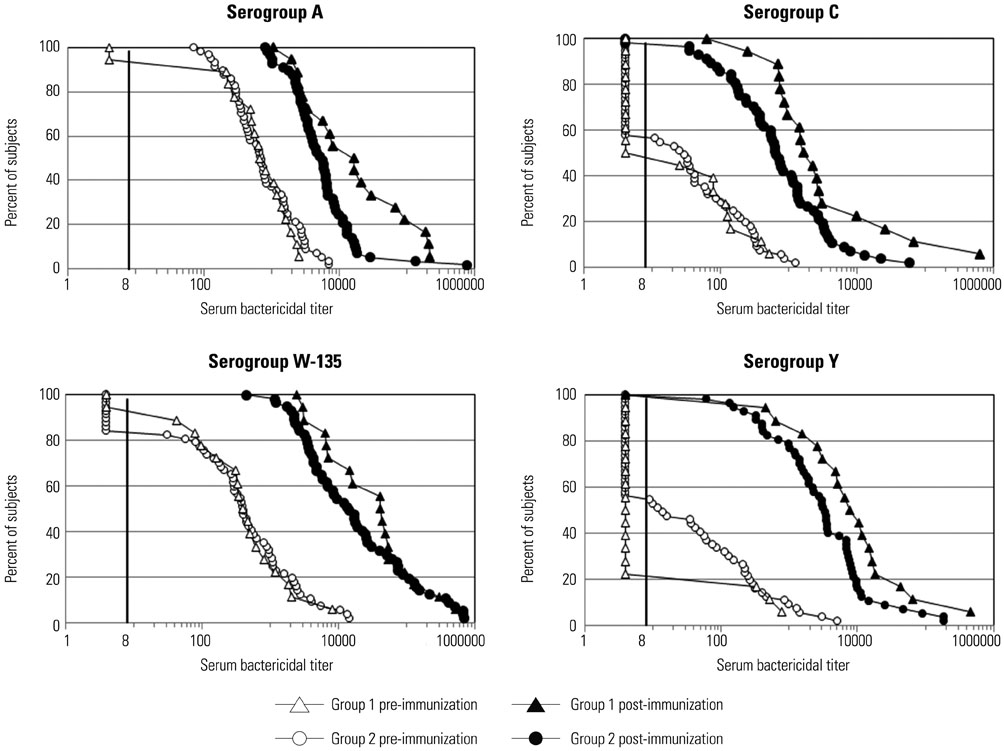
- The quadrivalent meningococcal conjugate vaccine (MenACWY-CRM) has been introduced for military recruits in Korea since 2012. This study was performed to evaluate the immunogenicity of MenACWY-CRM in Korean military recruits. In addition, the influence of tetanus-diphtheria toxoids (Td) vaccination on the vaccine response to MenACWY-CRM was analyzed. A total of 75 military recruits were enrolled. Among them, 18 received a dose of MenACWY-CRM only (group 1), and 57 received Td three days before MenACWY-CRM immunization (group 2). The immunogenicity of MenACWY-CRM was compared between the two groups. The serum bactericidal activity with baby rabbit complement was measured before and three weeks after immunization against serogroups A, C, W-135, and Y. The geometric mean titers (GMTs) against four serogroups were significantly increased in both groups after immunization. Compared to group 2, group 1 exhibited significantly higher vaccine responses in several aspects: post-immune GMTs against serogroup A and C, seroresponse rates against serogroup A, and a fold increases of titers against serogroup A, C, and Y. MenACWY-CRM was immunogenic against all vaccine-serogroups in Korean military recruits. Vaccine response to MenACWY-CRM was influenced by Td administered three days earlier.
MeSH Terms
-
Animals
Diphtheria Toxoid/chemistry/*immunology
Female
Humans
Immunization
Infant
Male
Meningococcal Infections/*prevention & control
Meningococcal Vaccines/administration & dosage/*immunology/therapeutic use
*Military Personnel
Rabbits
Republic of Korea
Serogroup
Serum Bactericidal Antibody Assay
Tetanus Toxoid/chemistry/*immunology
Time Factors
Treatment Outcome
*Vaccination
Vaccines, Conjugate/administration & dosage/immunology/therapeutic use
Diphtheria Toxoid
MenACWY
Meningococcal Vaccines
Tetanus Toxoid
Vaccines, Conjugate
Figure
Cited by 1 articles
-
Comparison of Immune Responses to Two Quadrivalent Meningococcal Conjugate Vaccines (CRM197 and Diphtheria Toxoid) in Healthy Adults
Han Wool Kim, Soyoung Lee, Ji Hyen Lee, So-Youn Woo, Kyung-Hyo Kim
J Korean Med Sci. 2019;34(23):. doi: 10.3346/jkms.2019.34.e169.
Reference
-
1. Harrison LH, Pass MA, Mendelsohn AB, Egri M, Rosenstein NE, Bustamante A, et al. Invasive meningococcal disease in adolescents and young adults. JAMA. 2001; 286:694–699.
Article2. Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med. 2001; 344:1378–1388.
Article3. Thompson MJ, Ninis N, Perera R, Mayon-White R, Phillips C, Bailey L, et al. Clinical recognition of meningococcal disease in children and adolescents. Lancet. 2006; 367:397–403.
Article4. Agrawal S, Nadel S. Acute bacterial meningitis in infants and children: epidemiology and management. Paediatr Drugs. 2011; 13:385–400.5. Broderick MP, Faix DJ, Hansen CJ, Blair PJ. Trends in meningococcal disease in the United States military, 1971-2010. Emerg Infect Dis. 2012; 18:1430–1437.
Article6. Meningococcal disease and college students. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000; 49:13–20.7. Park HS, Chun YI. Vaccination effect on pharyngeal carrier rate of Neisseria meningitidis and its serogroups in Korean Army recruits. J Korean Mil Med Assoc. 1992; 23:105–115.8. Hwang IU, Lee HK, Seo MY, Kim JP, Seo YB, Bang YJ. The changes of Meningococcal carriage rate and the serogroup in Korean army recruits. J Korean Mil Med Assoc. 2010; 41:188–199.9. Lee SO, Ryu SH, Park SJ, Ryu J, Woo JH, Kim YS. Meningococcal disease in the republic of Korea army: incidence and serogroups determined by PCR. J Korean Med Sci. 2003; 18:163–166.
Article10. Maslanka SE, Gheesling LL, Libutti DE, Donaldson KB, Harakeh HS, Dykes JK, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol. 1997; 4:156–167.
Article11. Andrews N, Borrow R, Miller E. Validation of serological correlate of protection for meningococcal C conjugate vaccine by using efficacy estimates from postlicensure surveillance in England. Clin Diagn Lab Immunol. 2003; 10:780–786.
Article12. Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969; 129:1307–1326.13. Jackson LA, Baxter R, Reisinger K, Karsten A, Shah J, Bedell L, et al. Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin Infect Dis. 2009; 49:e1–e10.
Article14. Lee HJ, Chung MH, Kim WJ, Hong YJ, Choi KM, Lee J, et al. Immunogenicity and safety of a novel quadrivalent meningococcal conjugate vaccine (MenACWY-CRM) in healthy Korean adolescents and adults. Int J Infect Dis. 2014; 28:204–210.
Article15. Reisinger KS, Baxter R, Block SL, Shah J, Bedell L, Dull PM. Quadrivalent meningococcal vaccination of adults: phase III comparison of an investigational conjugate vaccine, MenACWY-CRM, with the licensed vaccine, Menactra. Clin Vaccine Immunol. 2009; 16:1810–1815.
Article16. Kim SA, Kim DW, Dong BQ, Kim JS, Anh DD, Kilgore PE. An expanded age range for meningococcal meningitis: molecular diagnostic evidence from population-based surveillance in Asia. BMC Infect Dis. 2012; 12:310.
Article17. Bae SM, Kang YH. Serological and genetic characterization of meningococcal isolates in Korea. Jpn J Infect Dis. 2008; 61:434–437.18. Heo JY, Bae SM, Cheong HJ, Kim WJ, Kim MY, Na W, et al. Impact of quadrivalent meningococcal conjugate vaccine on carried meningococci in Korean Military trainee. J Korean Mil Med Assoc. 2014; 45:33–42.19. Glode MP, Robbins JB, Liu TY, Gotschlich EC, Orskov I, Orskov F. Cross-antigenicity and immunogenicity between capsular polysaccharides of group C Neisseria meningitidis and of Escherichia coli K92. J Infect Dis. 1977; 135:94–104.20. Gold R, Goldschneider I, Lepow ML, Draper TF, Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J Infect Dis. 1978; 137:112–121.
Article21. Arguedas A, Soley C, Loaiza C, Rincon G, Guevara S, Perez A, et al. Safety and immunogenicity of one dose of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, when administered to adolescents concomitantly or sequentially with Tdap and HPV vaccines. Vaccine. 2010; 28:3171–3179.
Article22. Gasparini R, Conversano M, Bona G, Gabutti G, Anemona A, Dull PM, et al. Randomized trial on the safety, tolerability, and immunogenicity of MenACWY-CRM, an investigational quadrivalent meningococcal glycoconjugate vaccine, administered concomitantly with a combined tetanus, reduced diphtheria, and acellular pertussis vaccine in adolescents and young adults. Clin Vaccine Immunol. 2010; 17:537–544.
Article23. Burrage M, Robinson A, Borrow R, Andrews N, Southern J, Findlow J, et al. Effect of vaccination with carrier protein on response to meningococcal C conjugate vaccines and value of different immunoassays as predictors of protection. Infect Immun. 2002; 70:4946–4954.
Article24. Pobre K, Tashani M, Ridda I, Rashid H, Wong M, Booy R. Carrier priming or suppression: understanding carrier priming enhancement of anti-polysaccharide antibody response to conjugate vaccines. Vaccine. 2014; 32:1423–1430.
Article25. Borrow R, Andrews N, Goldblatt D, Miller E. Serological basis for use of meningococcal serogroup C conjugate vaccines in the United Kingdom: reevaluation of correlates of protection. Infect Immun. 2001; 69:1568–1573.
Article26. Borrow R, Balmer P, Miller E. Meningococcal surrogates of protection--serum bactericidal antibody activity. Vaccine. 2005; 23:2222–2227.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Immune Responses to Two Quadrivalent Meningococcal Conjugate Vaccines (CRM197 and Diphtheria Toxoid) in Healthy Adults
- Antltoxln response to diphtheria and tetananus vaccine
- One-year antibody persistence and safety of a 4-dose schedule of MenACWY-CRM in healthy infants from South Korea
- Immunogenicity of Haemophilus influenzae Type b Conjugate Vaccines in Korean Infants: A Meta-analysis
- Immunogenicity and Safety of Diphtheria-tetanus Vaccine in Adults