Korean J Radiol.
2018 Feb;19(1):93-100. 10.3348/kjr.2018.19.1.93.
In Vivo Assessment of Neurodegeneration in Type C Niemann-Pick Disease by IDEAL-IQ
- Affiliations
-
- 1Department of Radiology, the Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou 510630, China. zhangyongzssy@126.com
- 2Department of Nuclear Medicine, the Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou 510630, China.
- 3Department of VIP Medical Center, the Third Affiliated Hospital, Sun Yat-Sen University, Guangzhou 510630, China.
- KMID: 2425114
- DOI: http://doi.org/10.3348/kjr.2018.19.1.93
Abstract
OBJECTIVE
To noninvasively assess the neurodegenerative changes in the brain of patients with Niemann-Pick type C (NPC) disease by measuring the lesion tissue with the iterative decomposition of water and fat with echo asymmetry and least square estimation-iron quantification (IDEAL-IQ).
MATERIALS AND METHODS
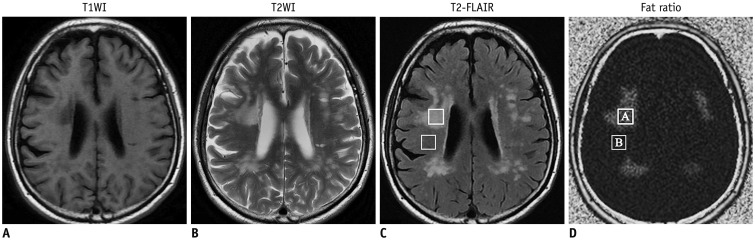
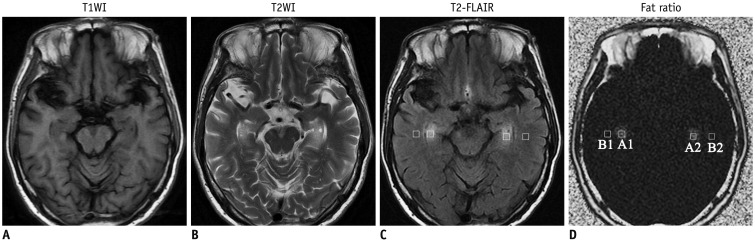
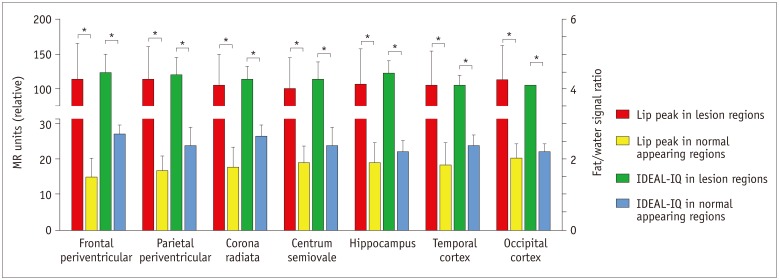
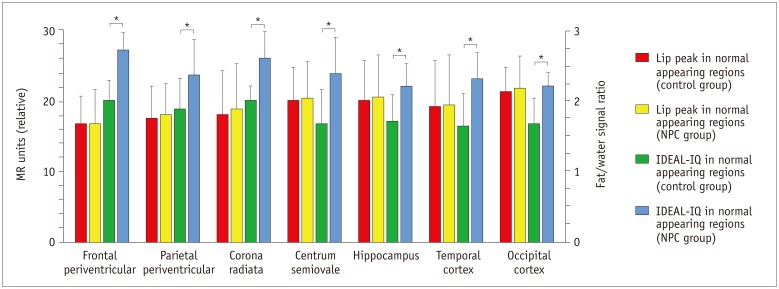
Routine brain MRI, IDEAL-IQ and 1H-proton magnetic resonance spectroscopy (1H-MRS, served as control) were performed on 12 patients with type C Niemann-Pick disease (4 males and 8 females; age range, 15-61 years; mean age, 36 years) and 20 healthy subjects (10 males and 10 females; age range, 20-65 years; mean age, 38 years). The regions with lesion and the normal appearing regions (NARs) of patients were measured and analyzed based on the fat/water signal intensity on IDEAL-IQ and the lipid peak on 1H-MRS.
RESULTS
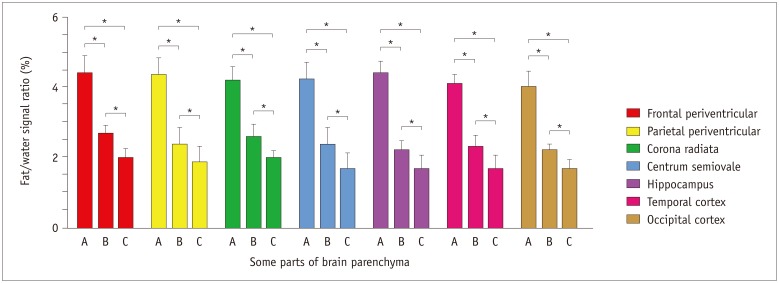
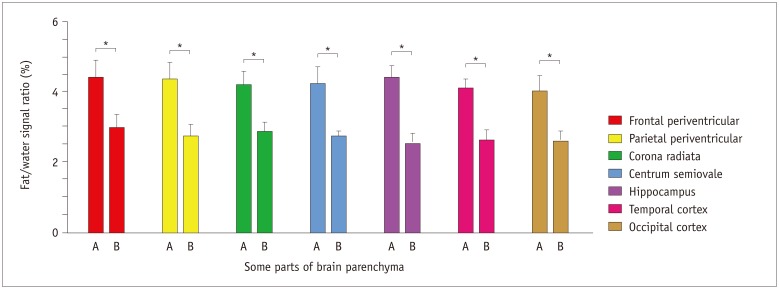
Niemann-Pick type C patients showed a higher fat/water signal intensity ratio with IDEAL-IQ on T2 hyperintensity lesions and NARs (3.7-4.9%, p < 0.05 and 1.8-3.0%, p < 0.05, respectively), as compared to healthy controls (HCs) (1.2-2.3%). After treatment, the fat/water signal intensity ratio decreased (2.2-3.4%), but remained higher than in the HCs (p < 0.05). The results of the 1H-MRS measurements showed increased lipid peaks in the same lesion regions, and the micro-lipid storage disorder of NARs in NPC patients was detectable by IDEAL-IQ instead of 1H-MRS.
CONCLUSION
The findings of this study suggested that IDEAL-IQ may be useful as a noninvasive and objective method in the evaluation of patients with NPC; additionally, IDEAL-IQ can be used to quantitatively measure the brain parenchymal adipose content and monitor patient follow-up after treatment of NPC.
Keyword
MeSH Terms
Figure
Reference
-
1. Yang CC, Su YN, Chiou PC, Fietz MJ, Yu CL, Hwu WL, et al. Six novel NPC1 mutations in Chinese patients with Niemann-Pick disease type C. J Neurol Neurosurg Psychiatry. 2005; 76:592–595. PMID: 15774455.
Article2. Brady RO, Filling-Katz MR, Barton NW, Pentchev PG. Niemann-Pick disease types C and D. Neurol Clin. 1989; 7:75–88. PMID: 2646522.
Article3. Fink JK, Filling-Katz MR, Sokol J, Cogan DG, Pikus A, Sonies B, et al. Clinical spectrum of Niemann-Pick disease type C. Neurology. 1989; 39:1040–1049. PMID: 2761697.
Article4. Vanier MT. Phenotypic and genetic heterogeneity in Niemann-Pick disease type C: current knowledge and practical implications. Wien Klin Wochenschr. 1997; 109:68–73. PMID: 9060145.5. Imrie J, Vijayaraghaven S, Whitehouse C, Harris S, Heptinstall L, Church H, et al. Niemann-Pick disease type C in adults. J Inherit Metab Dis. 2002; 25:491–500. PMID: 12555942.
Article6. Sévin M, Lesca G, Baumann N, Millat G, Lyon-Caen O, Vanier MT, et al. The adult form of Niemann-Pick disease type C. Brain. 2007; 130:120–133. PMID: 17003072.
Article7. Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004; 10:704–711. PMID: 15208706.
Article8. Park M, Moon WJ. Structural MR imaging in the diagnosis of Alzheimer's disease and other neurodegenerative dementia: current imaging approach and future perspectives. Korean J Radiol. 2016; 17:827–845. PMID: 27833399.
Article9. Ma J, Song Z, Yan F. Detection of hepatic and pancreatic fat infiltration in type II diabetes mellitus patients with IDEAL-Quant using 3.0T MR: comparison with single-voxel proton spectroscopy. Chin Med J (Engl). 2014; 127:3548–3552. PMID: 25316227.10. Kim H, Taksali SE, Dufour S, Befroy D, Goodman TR, Petersen KF, et al. Comparative MR study of hepatic fat quantification using single-voxel proton spectroscopy, two-point dixon and three-point IDEAL. Magn Reson Med. 2008; 59:521–527. PMID: 18306404.
Article11. Hofstetter LW, Yeo DT, Dixon WT, Kempf JG, Davis CE, Foo TK. Fat-referenced MR thermometry in the breast and prostate using IDEAL. J Magn Reson Imaging. 2012; 36:722–732. PMID: 22581513.
Article12. Tedeschi G, Bonavita S, Barton NW, Betolino A, Frank JA, Patronas NJ, et al. Proton magnetic resonance spectroscopic imaging in the clinical evaluation of patients with Niemann-Pick type C disease. J Neurol Neurosurg Psychiatry. 1998; 65:72–79. PMID: 9667565.
Article13. Galanaud D, Tourbah A, Lehéricy S, Leveque N, Heron B, Billette de Villemeur T, et al. 24 month-treatment with miglustat of three patients with Niemann-Pick disease type C: follow up using brain spectroscopy. Mol Genet Metab. 2009; 96:55–58. PMID: 19013089.
Article14. Sylvain M, Arnold DL, Scriver CR, Schreiber R, Shevell MI. Magnetic resonance spectroscopy in Niemann-Pick disease type C: correlation with diagnosis and clinical response to cholestyramine and lovastatin. Pediatr Neurol. 1994; 10:228–232. PMID: 8060425.
Article15. Zaaraoui W, Crespy L, Rico A, Faivre A, Soulier E, Confort-Gouny S, et al. In vivo quantification of brain injury in adult Niemann-Pick disease Type C. Mol Genet Metab. 2011; 103:138–141. PMID: 21397539.
Article16. Robertson DM, van Amelsvoort T, Daly E, Simmons A, Whitehead M, Morris RG, et al. Effects of estrogen replacement therapy on human brain aging: an in vivo 1H MRS study. Neurology. 2001; 57:2114–2117. PMID: 11739837.
Article17. Harzer K, Schlote W, Peiffer J, Benz HU, Anzil P. Neurovisceral lipidosis compatible with Niemann-Pick disease type C: morphological and biochemical studies of a late infantile case and enzyme and lipid assays in a prenatal case of the same family. Acta Neuropathol. 1978; 43:97–104. PMID: 209660.
Article18. Ong WY, Kumar U, Switzer RC, Sidhu A, Suresh G, Hu CY, et al. Neurodegeneration in Niemann-Pick type C disease mice. Exp Brain Res. 2001; 141:218–231. PMID: 11713633.
Article