Yonsei Med J.
2018 Nov;59(9):1079-1087. 10.3349/ymj.2018.59.9.1079.
Effects of Antioxidant Tempol on Systematic Inflammation and Endothelial Apoptosis in Emphysematous Rats Exposed to Intermittent Hypoxia
- Affiliations
-
- 1Respiratory Department of Tianjin Medical University General Hospital, Tianjin, China. tjzyyhxkcj@163.com, luckdonglixia@163.com
- 2Respiratory Department of Tianjin Medical University General Hospital Airport Hospital, Tianjin, China.
- 3Department of Life Sciences, State Key Laboratory of Medicinal Chemical Biology, College of Life Sciences, Nankai University, Tianjin, China.
- KMID: 2422493
- DOI: http://doi.org/10.3349/ymj.2018.59.9.1079
Abstract
- PURPOSE
Obstructive sleep apnea and chronic obstructive pulmonary disease are independent risk factors of cardiovascular disease (CVD), and their coexistence is known as overlap syndrome (OS). Endothelial dysfunction is the initial stage of CVD; however, underlying mechanisms linking OS and CVD are not well understood. The aim of this study was to explore whether OS can lead to more severe inflammation and endothelial apoptosis by promoting endothelial dysfunction, and to assess the intervention effects of antioxidant tempol.
MATERIALS AND METHODS
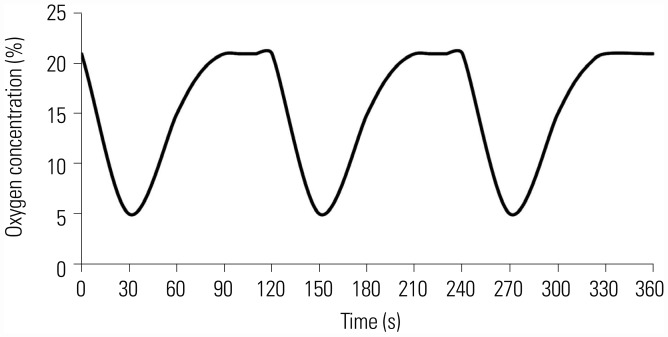
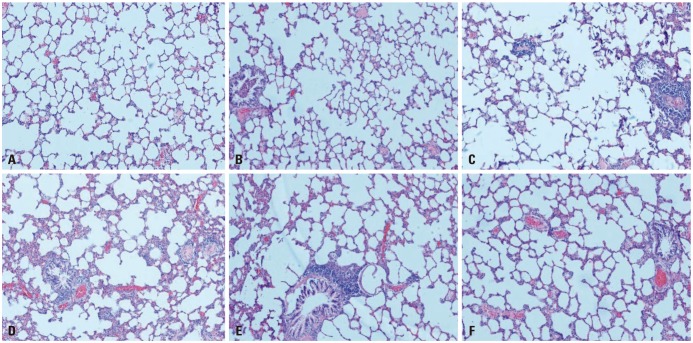
Male Wistar rats (n=66) were exposed to normal oxygen [normal control (NC) group], intermittent hypoxia (IH group), cigarette smoke (CH group), as well as cigarette smoke and IH (OS group). Tempol intervention was assessed in OS group treated with tempol (OST group) or NaCl (OSN group). After an 8-week challenge, lung tissues, serum, and fresh blood were harvested for analysis of endothelial markers and apoptosis.
RESULTS
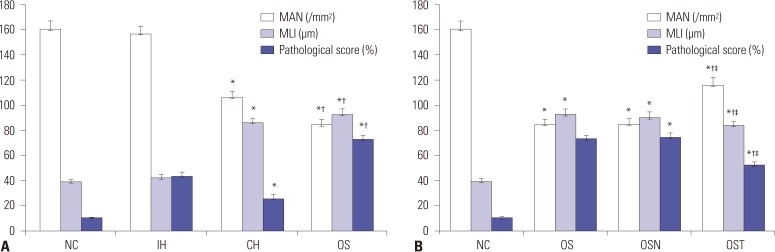
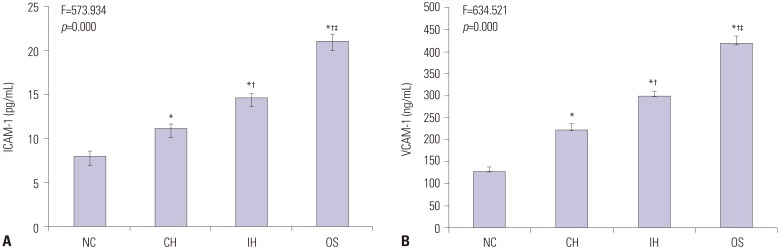
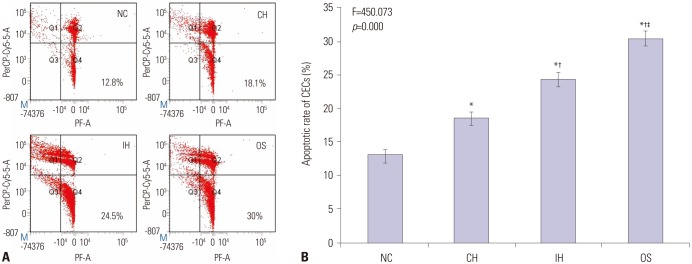
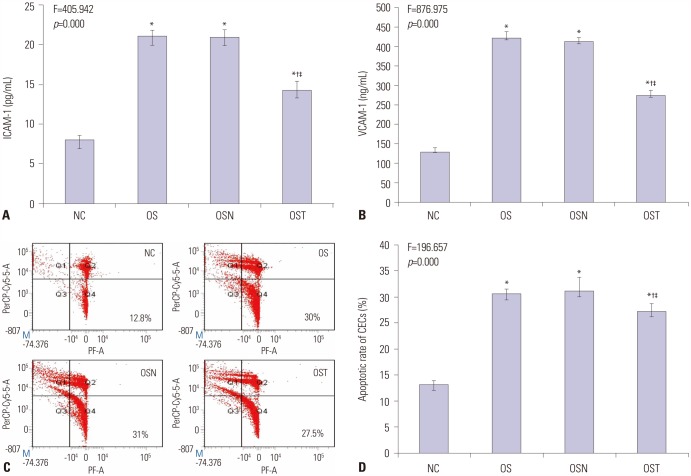
The levels of intracellular adhesion molecule-1, vascular cellular adhesion molecule-1, and apoptosis in circulating epithelial cells were the highest in OS group and the lowest in NC group. These levels were all greater in IH group than in CH group, and were lower in OST group than in OS and OSN groups (all p < 0.001).
CONCLUSION
Synergistic effects of IH with cigarette smoke-induced emphysema produce a greater inflammatory status and endothelial apoptosis. OS-related inflammation and endothelial cell apoptosis may play important roles in promoting cardiovascular dysfunction, and antioxidant tempol could achieve a partial protective effect.
Keyword
MeSH Terms
Figure
Reference
-
1. Li S, Feng J, Wei S, Qian X, Cao J, Chen B. Delayed neutrophil apoptosis mediates intermittent hypoxia-induced progressive heart failure in pressure-overloaded rats. Sleep Breath. 2016; 20:95–102. PMID: 26059543.
Article2. Turcani P, Skrickova J, Pavlik T, Janousova E, Orban M. The prevalence of obstructive sleep apnea in patients hospitalized for COPD exacerbation. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015; 159:422–428. PMID: 24572486.
Article3. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000; 342:1378–1384. PMID: 10805822.
Article4. Nakanishi K, Takeda Y, Tetsumoto S, Iwasaki T, Tsujino K, Kuhara H, et al. Involvement of endothelial apoptosis underlying chronic obstructive pulmonary disease-like phenotype in adiponectin-null mice: implications for therapy. Am J Respir Crit Care Med. 2011; 183:1164–1175. PMID: 21239691.5. Adeloye D, Chua S, Lee C, Basquill C, Papana A, Theodoratou E, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health. 2015; 5:020415. PMID: 26755942.
Article6. Maclay JD, MacNee W. Cardiovascular disease in COPD: mechanisms. Chest. 2013; 143:798–807. PMID: 23460157.7. McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnea: overlaps in pathophysiology, systemic inflammation, and cardiovascular disease. Am J Respir Crit Care Med. 2009; 180:692–700. PMID: 19628778.8. McNicholas WT. COPD-OSA overlap syndrome: evolving evidence regarding epidemiology, clinical consequences, and management. Chest. 2017; 152:1318–1326. PMID: 28442310.9. Ioachimescu OC, Teodorescu M. Integrating the overlap of obstructive lung disease and obstructive sleep apnoea: OLDOSA syndrome. Respirology. 2013; 18:421–431. PMID: 23368952.
Article10. Dragonieri S, Quaranta VN, Carratu P, Ranieri T, Resta O. Exhaled breath profiling in patients with COPD and OSA overlap syndrome: a pilot study. J Breath Res. 2016; 10:041001. PMID: 27811380.
Article11. Song D, Fang G, Greenberg H, Liu SF. Chronic intermittent hypoxia exposure-induced atherosclerosis: a brief review. Immunol Res. 2015; 63:121–130. PMID: 26407987.
Article12. Ambrosino P, Lupoli R, Iervolino S, De Felice A, Pappone N, Storino A, et al. Clinical assessment of endothelial function in patients with chronic obstructive pulmonary disease: a systematic review with meta-analysis. Intern Emerg Med. 2017; 12:877–885. PMID: 28593450.
Article13. Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest. 2015; 147:266–274. PMID: 25560865.14. Austin V, Crack PJ, Bozinovski S, Miller AA, Vlahos R. COPD and stroke: are systemic inflammation and oxidative stress the missing links? Clin Sci (Lond). 2016; 130:1039–1050. PMID: 27215677.
Article15. Jiang Y, Jiang LL, Maimaitirexiati XM, Zhang Y, Wu L. Irbesartan attenuates TNF-α-induced ICAM-1, VCAM-1, and E-selectin expression through suppression of NF-κB pathway in HUVECs. Eur Rev Med Pharmacol Sci. 2015; 19:3295–3302. PMID: 26400537.16. Lee CC, Huang SH, Yang YT, Cheng YW, Li CH, Kang JJ. Motorcycle exhaust particles up-regulate expression of vascular adhesion molecule-1 and intercellular adhesion molecule-1 in human umbilical vein endothelial cells. Toxicol In Vitro. 2012; 26:552–560. PMID: 22313677.
Article17. Xiao Y, Wang YC, Li LL, Jin YC, Sironi L, Wang Y, et al. Lactones from Ligusticum chuanxiong Hort. reduces atherosclerotic lesions in apoE-deficient mice via inhibiting over expression of NF-κB-dependent adhesion molecules. Fitoterapia. 2014; 95:240–246. PMID: 24594239.18. Liu X, Liu Y, Huang X, Lin G, Xie C. Endothelial progenitor cell dysfunction in acute exacerbation of chronic obstructive pulmonary disease. Mol Med Rep. 2017; 16:5294–5302. PMID: 28849108.
Article19. Chiang CH, Huang PH, Chung FP, Chen ZY, Leu HB, Huang CC, et al. Decreased circulating endothelial progenitor cell levels and function in patients with nonalcoholic fatty liver disease. PLoS One. 2012; 7:e31799. PMID: 22359630.
Article20. Feng J, Zhang D, Chen B. Endothelial mechanisms of endothelial dysfunction in patients with obstructive sleep apnea. Sleep Breath. 2012; 16:283–294. PMID: 21479903.
Article21. Ma L, Zhang J, Liu Y. Roles and mechanisms of obstructive sleep apnea-hypopnea syndrome and chronic intermittent hypoxia in atherosclerosis: evidence and prospective. Oxid Med Cell Longev. 2016; 2016:8215082. PMID: 27293515.
Article22. Simonsen U, Rodriguez-Rodriguez R, Dalsgaard T, Buus NH, Stankevicius E. Novel approaches to improving endothelium-dependent nitric oxide-mediated vasodilatation. Pharmacol Rep. 2009; 61:105–115. PMID: 19307698.
Article23. Cannizzo B, Quesada I, Militello R, Amaya C, Miatello R, Cruzado M, et al. Tempol attenuates atherosclerosis associated with metabolic syndrome via decreased vascular inflammation and NADPH-2 oxidase expression. Free Radic Res. 2014; 48:526–533. PMID: 24490696.
Article24. Dumitrascu R, Heitmann J, Seeger W, Weissmann N, Schulz R. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid Med Cell Longev. 2013; 2013:234631. PMID: 23533685.
Article25. Li C, Yang X, Feng J, Lei P, Wang Y. Proinflammatory and prothrombotic status in emphysematous rats exposed to intermittent hypoxia. Int J Clin Exp Pathol. 2015; 8:374–383. PMID: 25755725.26. Guo H, Cao J, Li J, Yang X, Jiang J, Feng J, et al. Lymphocytes from intermittent hypoxia-exposed rats increase the apoptotic signals in endothelial cells via oxidative and inflammatory injury in vitro. Sleep Breath. 2015; 19:969–976. PMID: 25637094.
Article27. Feng J, Wang QS, Chiang A, Chen BY. The effects of sleep hypoxia on coagulant factors and hepatic inflammation in emphysematous rats. PLoS One. 2010; 5:e13201. PMID: 20949089.28. Chunhua C, Bing H, Xiuying T, Hong Z. Study on the pathogenesis of chronic obstructive pulmonary disease induced by tobacco smoke. The changes of Clara cells' structure and Clara cell secretory protein in rats. J Cardiovasc Pulmon Dis. 2000; 19:224–227.29. Yang QC, Sun X, Wang YM, Wu Q, Feng J, Chen BY. Systematic and endothelial inflammation and endothelial progenitor cell levels in emphysematous rats exposed to intermittent hypoxia. Respir Care. 2015; 60:279–289. PMID: 25587169.
Article30. Lavie L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia--revisited--the bad ugly and good: implications to the heart and brain. Sleep Med Rev. 2015; 20:27–45. PMID: 25155182.31. McNicholas WT. Chronic obstructive pulmonary disease and obstructive sleep apnoea-the overlap syndrome. J Thorac Dis. 2016; 8:236–242. PMID: 26904264.32. El-Solh AA, Mador MJ, Sikka P, Dhillon RS, Amsterdam D, Grant BJ. Adhesion molecules in patients with coronary artery disease and moderate-to-severe obstructive sleep apnea. Chest. 2002; 121:1541–1547. PMID: 12006441.
Article33. Ursavas¸ A, KaradagXMLLink_XYZ M, Rodoplu E, Yilmaztepe A, Oral HB, Gözü RO. Circulating ICAM-1 and VCAM-1 levels in patients with obstructive sleep apnea syndrome. Respiration. 2007; 74:525–532. PMID: 17148932.
Article34. El-Deek SE, Makhlouf HA, Saleem TH, Mandour MA, Mohamed NA. Surfactant protein D, soluble intercellular adhesion molecule-1 and high-sensitivity C-reactive protein as biomarkers of chronic obstructive pulmonary disease. Med Princ Pract. 2013; 22:469–474. PMID: 23860258.
Article35. Zhang BY, Jin Z, Zhao Z. Long intergenic noncoding RNA 00305 sponges miR-136 to regulate the hypoxia induced apoptosis of vascular endothelial cells. Biomed Pharmacother. 2017; 94:238–243. PMID: 28763747.
Article36. El Solh AA, Akinnusi ME, Baddoura FH, Mankowski CR. Endothelial cell apoptosis in obstructive sleep apnea: a link to endothelial dysfunction. Am J Respir Crit Care Med. 2007; 175:1186–1191. PMID: 17272785.37. Nana-Sinkam SP, Lee JD, Sotto-Santiago S, Stearman RS, Keith RL, Choudhury Q, et al. Prostacyclin prevents pulmonary endothelial cell apoptosis induced by cigarette smoke. Am J Respir Crit Care Med. 2007; 175:676–685. PMID: 17255567.
Article38. Zhu F, Wang Q, Guo C, Wang X, Cao X, Shi Y, et al. IL-17 induces apoptosis of vascular endothelial cells: a potential mechanism for human acute coronary syndrome. Clin Immunol. 2011; 141:152–160. PMID: 21872532.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reoxygenation stimulates EDRF(s) release from endothelial cells of rabbit aorta
- Effects of Tempol on Blood Pressure and Tissue Oxidative Stress in DOCA-alt and L-AME-nduced Hypertension
- Melatonin mitigates the adverse effect of hypoxia during myocardial differentiation in mouse embryonic stem cells
- Effect of Vitamin E on Cerebral Hypoxia-Ischemia in Neonatal Rats
- Neonatal Rat Necrotizing Enterocolitis Model Adopting Oral Endotoxin and Hypoxia Exhibits Increased Apoptosis through Caspase-3 Activation