J Korean Soc Transplant.
2018 Sep;32(3):57-62. 10.4285/jkstn.2018.32.3.57.
Bortezomib Treatment for Refractory Antibody-Mediated Rejection Superimposed with BK Virus-Associated Nephropathy during the Progression of Recurrent C3 Glomerulonephritis
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Kyungpook National University, Daegu, Korea.
- 2Department of Internal Medicine, Daegu Fatima Hospital, Daegu, Korea.
- 3Department of Pathology, Yeungnam University Medical Center, Yeungnam University College of Medicine, Daegu, Korea.
- 4Department of Pathology, School of Medicine, Kyungpook National University, Daegu, Korea. yyjjkim@ynu.ac.kr
- KMID: 2421281
- DOI: http://doi.org/10.4285/jkstn.2018.32.3.57
Abstract
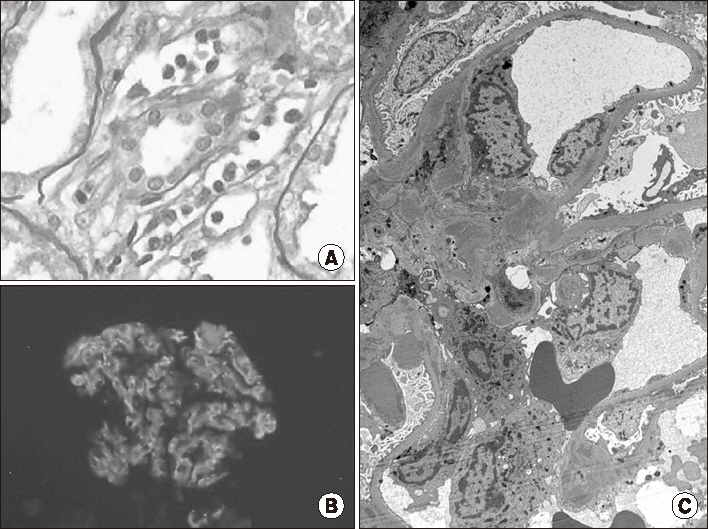
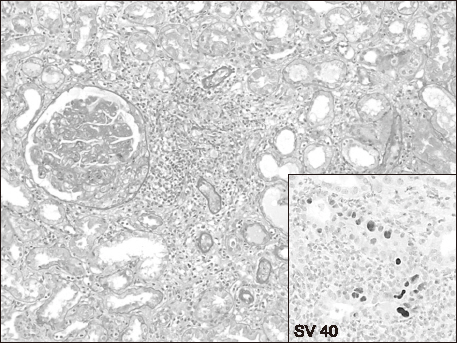
- A 38-year-old man, who underwent a second kidney transplantation (KT), was admitted because of antibody-mediated rejection (AMR) complicated by BK virus-associated nephropathy (BKVAN). He was placed on hemodialysis at the age of 24 years because of membranoproliferative glomerulonephritis. At the age of 28 years, he underwent a living donor KT from his father; however, 1 year after the transplantation, he developed a recurrence of the primary glomerular disease, resulting in graft failure 2 years after the first KT. Ten years later, he received a deceased-donor kidney with a B-cell-positive-cross-match. He received 600 mg of rituximab before the KT with three cycles of plasmapheresis and immunoglobulin (0.5 g/kg) therapy after KT. During the follow-up, the first and second allograft biopsies at 4 and 10 months after KT revealed AMR with a recurrence of primary glomerular disease that was reclassified as C3 glomerulonephritis (C3GN). He received a steroid pulse, rituximab, plasmapheresis, and immunoglobulin therapies. The third allograft biopsy demonstrated that the BKVAN was complicated with AMR and C3GN. As the azotemia did not improve after repeated conventional therapies for AMR, one cycle of bortezomib (1.3 mg/m²Ã—4 doses) was administered. The allograft function stabilized, and BK viremia became undetectable after 6 months. The present case suggests that bortezomib therapy may be applicable to patients with refractory AMR, even in cases complicated with BKVAN.
Keyword
MeSH Terms
-
Adult
Allografts
Azotemia
Biopsy
BK Virus
Bortezomib*
Fathers
Follow-Up Studies
Glomerulonephritis*
Glomerulonephritis, Membranoproliferative
Graft Rejection
Humans
Immunization, Passive
Immunoglobulins
Kidney
Kidney Transplantation
Living Donors
Plasmapheresis
Recurrence
Renal Dialysis
Rituximab
Transplants
Viremia
Bortezomib
Immunoglobulins
Rituximab
Figure
Reference
-
1. Sellares J, de Freitas DG, Mengel M, Reeve J, Einecke G, Sis B, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant. 2012; 12:388–399.
Article2. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009; 9:Suppl 3. S1–155.3. Archdeacon P, Chan M, Neuland C, Velidedeoglu E, Meyer J, Tracy L, et al. Summary of FDA antibody-mediated rejection workshop. Am J Transplant. 2011; 11:896–906.
Article4. Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody-mediated rejection in kidney transplant recipients: a systematic review. Transplantation. 2012; 94:775–783.
Article5. Chung BH, Yang CW. Current issues in the treatment of chronic antibody-mediated rejection in kidney transplantation. Hanyang Med Rev. 2014; 34:211–216.
Article6. Perry DK, Burns JM, Pollinger HS, Amiot BP, Gloor JM, Gores GJ, et al. Proteasome inhibition causes apoptosis of normal human plasma cells preventing alloantibody production. Am J Transplant. 2009; 9:201–209.
Article7. Everly MJ, Everly JJ, Susskind B, Brailey P, Arend LJ, Alloway RR, et al. Bortezomib provides effective therapy for antibody-and cell-mediated acute rejection. Transplantation. 2008; 86:1754–1761.
Article8. Yang KS, Jeon H, Park Y, Jo IH, Kim JI, Moon IS, et al. Use of bortezomib as anti-humoral therapy in kidney transplantation. J Korean Med Sci. 2014; 29:648–651.
Article9. De Sousa-Amorim E, Revuelta I, Diekmann F, Cofan F, Lozano M, Cid J, et al. Bortezomib for refractory acute antibody-mediated rejection in kidney transplant recipients: a single-centre case series. Nephrology (Carlton). 2016; 21:700–704.
Article10. Lee J, Kim BS, Park Y, Lee JG, Lim BJ, Jeong HJ, et al. The effect of bortezomib on antibody-mediated rejection after kidney transplantation. Yonsei Med J. 2015; 56:1638–1642.
Article11. Lee HM, Jang IA, Lee D, Kang EJ, Choi BS, Park CW, et al. Risk factors in the progression of BK virus-associated nephropathy in renal transplant recipients. Korean J Intern Med. 2015; 30:865–872.
Article12. Kim YJ, Jeong JC, Koo TY, Kwon HY, Han M, Jeon HJ, et al. Impact of combined acute rejection on BK virus-associated nephropathy in kidney transplantation. J Korean Med Sci. 2013; 28:1711–1715.
Article13. McGregor SM, Chon WJ, Kim L, Chang A, Meehan SM. Clinical and pathological features of kidney transplant patients with concurrent polyomavirus nephropathy and rejection-associated endarteritis. World J Transplant. 2015; 5:292–299.
Article14. Nishio-Lucar A, Balogun RA, Sanoff S. Therapeutic apheresis in kidney transplantation: a review of renal transplant immunobiology and current interventions with apheresis medicine. J Clin Apher. 2013; 28:56–63.
Article15. Jordan S, Cunningham-Rundles C, McEwan R. Utility of intravenous immune globulin in kidney transplantation: efficacy, safety, and cost implications. Am J Transplant. 2003; 3:653–664.
Article16. Jordan SC, Vo AA, Peng A, Toyoda M, Tyan D. Intravenous gammaglobulin (IVIG): a novel approach to improve transplant rates and outcomes in highly HLA-sensitized patients. Am J Transplant. 2006; 6:459–466.
Article17. Wan SS, Ying TD, Wyburn K, Roberts DM, Wyld M, Chadban SJ. The treatment of antibody-mediated rejection in kidney transplantation: an updated systematic review and meta-analysis. Transplantation. 2018; 102:557–568.18. Sautenet B, Blancho G, Buchler M, Morelon E, Toupance O, Barrou B, et al. One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation: RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation. 2016; 100:391–399.
Article19. Woodle ES, Tremblay S, Driscoll J. Targeting plasma cells with proteasome inhibitors: principles from primates. J Am Soc Nephrol. 2017; 28:1951–1953.
Article20. Kim MG, Kim YJ, Kwon HY, Park HC, Koo TY, Jeong JC, et al. Outcomes of combination therapy for chronic antibody-mediated rejection in renal transplantation. Nephrology (Carlton). 2013; 18:820–826.
Article21. Walsh RC, Alloway RR, Girnita AL, Woodle ES. Proteasome inhibitor-based therapy for antibody-mediated rejection. Kidney Int. 2012; 81:1067–1074.
Article22. Woodle ES, Shields AR, Ejaz NS, Sadaka B, Girnita A, Walsh RC, et al. Prospective iterative trial of proteasome inhibitorbased desensitization. Am J Transplant. 2015; 15:101–118.
Article23. Diwan TS, Raghavaiah S, Burns JM, Kremers WK, Gloor JM, Stegall MD. The impact of proteasome inhibition on alloantibody-producing plasma cells in vivo. Transplantation. 2011; 91:536–541.
Article24. Gonzalez S, Escobar-Serna DP, Suarez O, Benavides X, Escobar-Serna JF, Lozano E. BK virus nephropathy in kidney transplantation: an approach proposal and update on risk factors, diagnosis, and treatment. Transplant Proc. 2015; 47:1777–1785.
Article25. Hodur DM, Mandelbrot D. Immunosuppression and BKV Nephropathy. N Engl J Med. 2002; 347:2079–2080.
Article26. Prince O, Savic S, Dickenmann M, Steiger J, Bubendorf L, Mihatsch MJ. Risk factors for polyoma virus nephropathy. Nephrol Dial Transplant. 2009; 24:1024–1033.
Article27. O'Leary JG, Samaniego M, Barrio MC, Potena L, Zeevi A, Djamali A, et al. The influence of immunosuppressive agents on the risk of de novo donor-specific HLA antibody production in solid organ transplant recipients. Transplantation. 2016; 100:39–53.28. Nickeleit V, Mihatsch MJ. Polyomavirus allograft nephropathy and concurrent acute rejection: a diagnostic and therapeutic challenge. Am J Transplant. 2004; 4:838–839.
Article29. Wu D, Zhang MC, Chen JS, Li X, Cheng DR, Xie KN, et al. BK virus-associated nephropathy with plasma cell-rich infiltrates treated by bortezomib-based regimen. Exp Clin Transplant. 2015; 13:603–606.30. Everly MJ. A summary of bortezomib use in transplantation across 29 centers. Clin Transpl. 2009; 23:323–337.31. Cosio FG, Cattran DC. Recent advances in our understanding of recurrent primary glomerulonephritis after kidney transplantation. Kidney Int. 2017; 91:304–314.
Article32. Blosser CD, Bloom RD. Recurrent glomerular disease after kidney transplantation. Curr Opin Nephrol Hypertens. 2017; 26:501–508.
Article33. Medjeral-Thomas NR, O'Shaughnessy MM, O'Regan JA, Traynor C, Flanagan M, Wong L, et al. C3 glomerulopathy: clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol. 2014; 9:46–53.
Article34. Zand L, Lorenz EC, Cosio FG, Fervenza FC, Nasr SH, Gandhi MJ, et al. Clinical findings, pathology, and outcomes of C3GN after kidney transplantation. J Am Soc Nephrol. 2014; 25:1110–1117.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of BK Virus Nephropathy with Strong C4d Deposition in a Renal Allograft Recipient
- The Effect of Bortezomib on the Management of Immediate Postoperative Refractory Antibody-Mediated Rejection after Kidney Transplantation
- Treatment of Posttransplantation Recurrent Glomerulonephritis: IgA Nephropathy, Membranous Nephropathy, Membranoproliferative Glomerulonephritis
- Treatment of Refractory Antibody-mediated Rejection with Bortezomib in a Kidney Transplant Recipient: A Case Report
- Effect of High Dose Intravenous Immunoglobulins on the Treatment of Antibody Mediated Humoral Rejection and BK Virus Infection in Renal Transplant Recipients