Korean Circ J.
2018 Oct;48(10):890-905. 10.4070/kcj.2018.0268.
Catheter Ablation of Ventricular Tachycardia in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy
- Affiliations
-
- 1Heart Rhythm Center, Division of Cardiology, Department of Medicine, Taipei Veterans General Hospital, Taipei, Taiwan. epsachen@ms41.hinet.net
- 2Institute of Clinical Medicine, Cardiovascular Research Center, National Yang-Ming University, Taipei, Taiwan.
- 3Department of Internal Medicine, Taipei Veterans General Hospital, Yuan-Shan Branch, I-LAN, Taiwan.
- KMID: 2420641
- DOI: http://doi.org/10.4070/kcj.2018.0268
Abstract
- Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is predominantly an inherited cardiomyopathy with typical histopathological characteristics of fibro-fatty infiltration mainly involving the right ventricular (RV) inflow tract, RV outflow tract, and RV apex in the majority of patients. The above pathologic evolution frequently brings patients with ARVD/C to medical attention owing to the manifestation of syncope, sudden cardiac death (SCD), ventricular arrhythmogenesis, or heart failure. To prevent future or recurrent SCD, an implantable cardiac defibrillator (ICD) is highly desirable in patients with ARVD/C who had experienced unexplained syncope, hemodynamically intolerable ventricular tachycardia (VT), ventricular fibrillation, and/or aborted SCD. Notably, the management of frequent ventricular tachyarrhythmias in ARVD/C is challenging, and the use of antiarrhythmic drugs could be unsatisfactory or limited by the unfavorable side effects. Therefore, radiofrequency catheter ablation (RFCA) has been implemented to treat the drug-refractory VT in ARVD/C for decades. However, the initial understanding of the link between fibro-fatty pathogenesis and ventricular arrhythmogenesis in ARVD/C is scarce, the efficacy and prognosis of endocardial RFCA alone were limited and disappointing. The electrophysiologists had broken through this frontier after better illustration of epicardial substrates and broadly application of epicardial approaches in ARVD/C. In recent works of literature, the application of epicardial ablation also successfully results in higher procedural success and decreases VT recurrences in patients with ARVD/C who are refractory to the endocardial approach during long-term follow-up. In this article, we review the important evolution on the delineation of arrhythmogenic substrates, ablation strategies, and ablation outcome of VT in patients with ARVD/C.
Keyword
MeSH Terms
-
Anti-Arrhythmia Agents
Arrhythmogenic Right Ventricular Dysplasia
Cardiomyopathies
Catheter Ablation*
Catheters*
Death, Sudden, Cardiac
Defibrillators
Epicardial Mapping
Follow-Up Studies
Heart Failure
Humans
Prognosis
Recurrence
Syncope
Tachycardia
Tachycardia, Ventricular*
Ventricular Fibrillation
Anti-Arrhythmia Agents
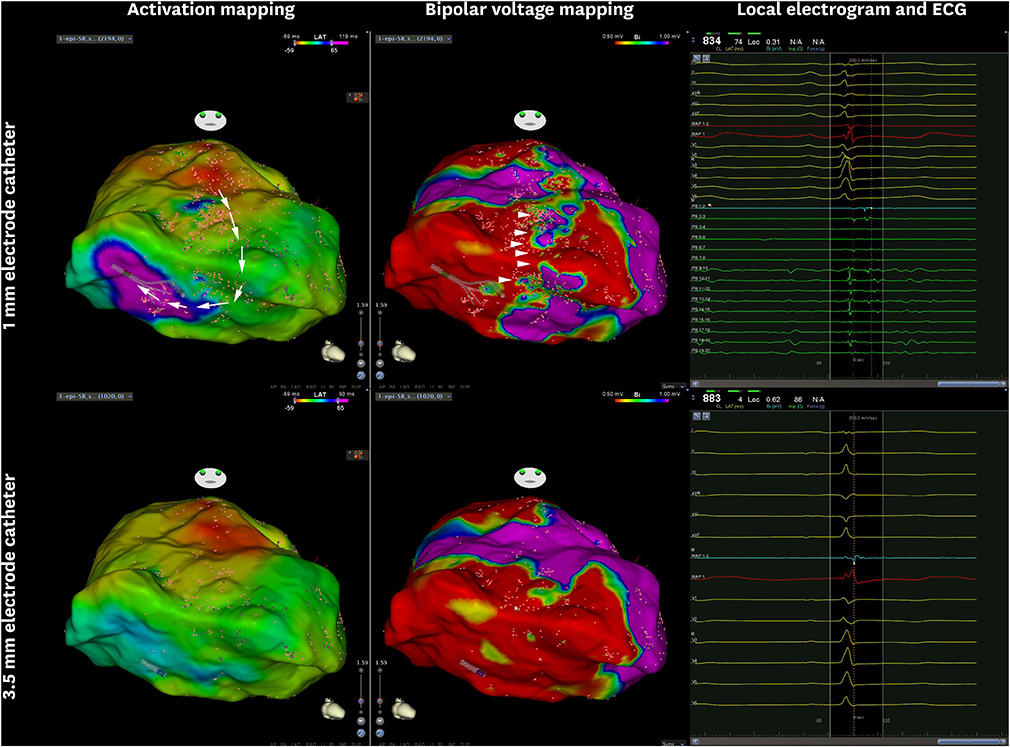
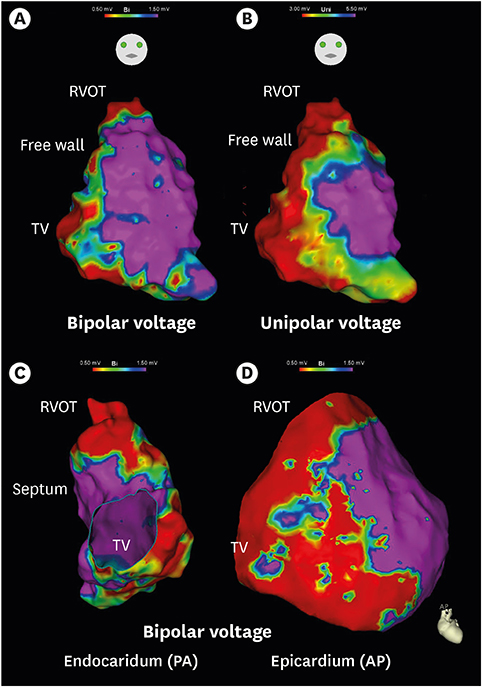
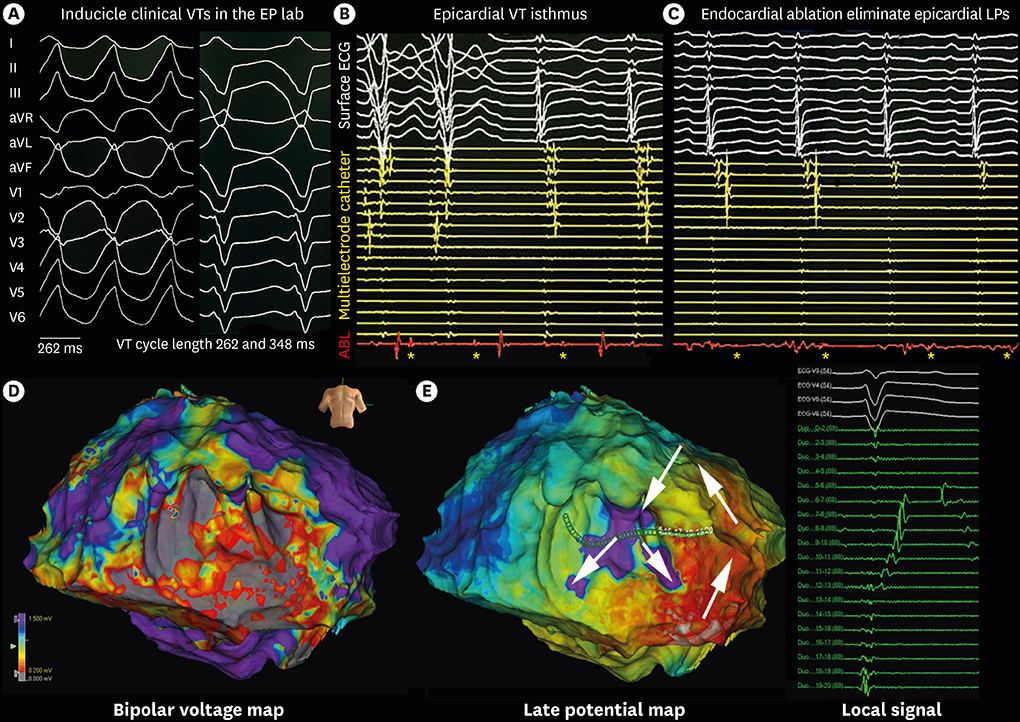
Figure
Reference
-
1. Dalla Volta S, Fameli O, Maschio G. The clinical and hemodynamic syndrome of auricularisation of the right ventricle. (Apropos of 4 personal cases). Arch Mal Coeur Vaiss. 1965; 58:1129–1143.2. Basso C, Thiene G, Corrado D, et al. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996; 94:983–991.3. Marcus FI, Fontaine GH, Guiraudon G, et al. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982; 65:384–398.
Article4. Hulot JS, Jouven X, Empana JP, et al. Natural history and risk stratification of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circulation. 2004; 110:1879–1884.
Article5. Dalal D, Nasir K, Bomma C, et al. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005; 112:3823–3832.
Article6. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Eur Heart J. 2010; 31:806–814.7. Corrado D, Wichter T, Link MS, et al. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation. 2015; 132:441–453.8. Maron BJ, Udelson JE, Bonow RO, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. 2015; 66:2362–2371.9. Corrado D, Leoni L, Link MS, et al. Implantable cardioverter-defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003; 108:3084–3091.
Article10. Wichter T, Paul M, Wollmann C, et al. Implantable cardioverter/defibrillator therapy in arrhythmogenic right ventricular cardiomyopathy: single-center experience of long-term follow-up and complications in 60 patients. Circulation. 2004; 109:1503–1508.
Article11. Marchlinski FE, Zado E, Dixit S, et al. Electroanatomic substrate and outcome of catheter ablative therapy for ventricular tachycardia in setting of right ventricular cardiomyopathy. Circulation. 2004; 110:2293–2298.
Article12. Garcia FC, Bazan V, Zado ES, et al. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009; 120:366–375.
Article13. Philips B, Madhavan S, James C, et al. Outcomes of catheter ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ Arrhythm Electrophysiol. 2012; 5:499–505.
Article14. Lin CY, Chung FP, Lin YJ, et al. Safety and efficacy of epicardial ablation of ventricular tachyarrhythmias: experience from a tertiary referral center in Taiwan. Acta Cardiol Sin. 2018; 34:49–58.15. Lin CY, Lin YJ, Li CH, et al. Heterogeneous distribution of substrates between the endocardium and epicardium promotes ventricular fibrillation in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Europace. 2018; 20:501–511.
Article16. Philips B, te Riele AS, Sawant A, et al. Outcomes and ventricular tachycardia recurrence characteristics after epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. Heart Rhythm. 2015; 12:716–725.
Article17. Santangeli P, Zado ES, Supple GE, et al. Long-term outcome with catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2015; 8:1413–1421.
Article18. McKenna WJ, Thiene G, Nava A, et al. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task force of the working group myocardial and pericardial disease of the European society of cardiology and of the scientific council on cardiomyopathies of the international society and federation of cardiology. Br Heart J. 1994; 71:215–218.
Article19. Avella A, d'Amati G, Pappalardo A, et al. Diagnostic value of endomyocardial biopsy guided by electroanatomic voltage mapping in arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Cardiovasc Electrophysiol. 2008; 19:1127–1134.
Article20. Chung FP, Lin YJ, Kuo L, et al. Catheter ablation of ventricular tachycardia/fibrillation in a patient with right ventricular amyloidosis with initial manifestations mimicking arrhythmogenic right ventricular dysplasia/cardiomyopathy. Korean Circ J. 2017; 47:282–285.
Article21. Philips B, Madhavan S, James CA, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy and cardiac sarcoidosis: distinguishing features when the diagnosis is unclear. Circ Arrhythm Electrophysiol. 2014; 7:230–236.22. Groeneweg JA, Bhonsale A, James CA, et al. Clinical presentation, long-term follow-up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet. 2015; 8:437–446.23. Corrado D, Calkins H, Link MS, et al. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation. 2010; 122:1144–1152.
Article24. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010; 121:1533–1541.25. Priori SG, Blomström-Lundqvist C, Mazzanti A, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace. 2015; 17:1601–1687.26. Romero J, Di Biase L, Diaz JC, et al. Early versus late referral for catheter ablation of ventricular tachycardia in patients with structural heart disease: a systematic review and meta-analysis of clinical outcomes. JACC Clin Electrophysiol. 2018; 4:374–382.27. Do VB, Tsai WC, Lin YJ, et al. The Different substrate characteristics of arrhythmogenic triggers in idiopathic right ventricular outflow tract tachycardia and arrhythmogenic right ventricular dysplasia: new insight from noncontact mapping. PLoS One. 2015; 10:e0140167.
Article28. Denis A, Sacher F, Derval N, et al. Diagnostic value of isoproterenol testing in arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2014; 7:590–597.
Article29. Bhonsale A, James CA, Tichnell C, et al. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter-defibrillator implantation for primary prevention. J Am Coll Cardiol. 2011; 58:1485–1496.
Article30. Saguner AM, Medeiros-Domingo A, Schwyzer MA, et al. Usefulness of inducible ventricular tachycardia to predict long-term adverse outcomes in arrhythmogenic right ventricular cardiomyopathy. Am J Cardiol. 2013; 111:250–257.
Article31. Link MS, Laidlaw D, Polonsky B, et al. Ventricular arrhythmias in the North American multidisciplinary study of ARVC: predictors, characteristics, and treatment. J Am Coll Cardiol. 2014; 64:119–125.32. Bai R, Di Biase L, Shivkumar K, et al. Ablation of ventricular arrhythmias in arrhythmogenic right ventricular dysplasia/cardiomyopathy: arrhythmia-free survival after endo-epicardial substrate based mapping and ablation. Circ Arrhythm Electrophysiol. 2011; 4:478–485.33. Tschabrunn CM, Roujol S, Dorman NC, et al. High-resolution mapping of ventricular scar: comparison between single and multielectrode catheters. Circ Arrhythm Electrophysiol. 2016; 9:e003841.34. Maagh P, Christoph A, Dopp H, et al. High-density mapping in ventricular tachycardia ablation: a PentaRay® study. Cardiol Res. 2017; 8:293–303.35. Aliot EM, Stevenson WG, Almendral-Garrote JM, et al. EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a registered branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm. 2009; 6:886–933.
Article36. Lin CY, Chung FP, Lin YJ, et al. Gender differences in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy: clinical manifestations, electrophysiological properties, substrate characteristics, and prognosis of radiofrequency catheter ablation. Int J Cardiol. 2017; 227:930–937.
Article37. Polin GM, Haqqani H, Tzou W, et al. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011; 8:76–83.
Article38. Cano O, Hutchinson M, Lin D, et al. Electroanatomic substrate and ablation outcome for suspected epicardial ventricular tachycardia in left ventricular nonischemic cardiomyopathy. J Am Coll Cardiol. 2009; 54:799–808.
Article39. Lin CY, Silberbauer J, Lin YJ, et al. Simultaneous amplitude frequency electrogram transformation (SAFE-T) mapping to identify ventricular tachycardia arrhythmogenic potentials in sinus rhythm. JACC Clin Electrophysiol. 2016; 2:459–470.40. Arenal A, del Castillo S, Gonzalez-Torrecilla E, et al. Tachycardia-related channel in the scar tissue in patients with sustained monomorphic ventricular tachycardias: influence of the voltage scar definition. Circulation. 2004; 110:2568–2574.41. Venlet J, Piers SR, Kapel GF, et al. Unipolar endocardial voltage mapping in the right ventricle: optimal cutoff values correcting for computed tomography-derived epicardial fat thickness and their clinical value for substrate delineation. Circ Arrhythm Electrophysiol. 2017; 10:e005175.
Article42. Wijnmaalen AP, van der Geest RJ, van Huls van Taxis CF, et al. Head-to-head comparison of contrast-enhanced magnetic resonance imaging and electroanatomical voltage mapping to assess post-infarct scar characteristics in patients with ventricular tachycardias: real-time image integration and reversed registration. Eur Heart J. 2011; 32:104–114.
Article43. Haqqani HM, Tschabrunn CM, Betensky BP, et al. Layered activation of epicardial scar in arrhythmogenic right ventricular dysplasia: possible substrate for confined epicardial circuits. Circ Arrhythm Electrophysiol. 2012; 5:796–803.44. Komatsu Y, Daly M, Sacher F, et al. Endocardial ablation to eliminate epicardial arrhythmia substrate in scar-related ventricular tachycardia. J Am Coll Cardiol. 2014; 63:1416–1426.
Article45. Tanawuttiwat T, Te Riele AS, Philips B, et al. Electroanatomic correlates of depolarization abnormalities in arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Cardiovasc Electrophysiol. 2016; 27:443–452.
Article46. Tschabrunn CM, Haqqani HM, Santangeli P, et al. 12-lead electrocardiogram to localize region of abnormal electroanatomic substrate in arrhythmogenic right ventricular cardiomyopathy. JACC Clin Electrophysiol. 2017; 3:654–665.47. Kubala M, Pathak RK, Xie S, et al. Electrocardiographic repolarization abnormalities and electroanatomic substrate in arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2018; 11:e005553.
Article48. Hsieh WH, Lin CY, Te AL, et al. A novel noninvasive surface ECG analysis using interlead QRS dispersion in arrhythmogenic right ventricular cardiomyopathy. PLoS One. 2017; 12:e0182364.
Article49. Bazan V, Bala R, Garcia FC, et al. Twelve-lead ECG features to identify ventricular tachycardia arising from the epicardial right ventricle. Heart Rhythm. 2006; 3:1132–1139.
Article50. Tokuda M, Tedrow UB, Inada K, et al. Direct comparison of adjacent endocardial and epicardial electrograms: implications for substrate mapping. J Am Heart Assoc. 2013; 2:e000215.
Article51. Chi PC, Lin YJ, Chang SL, et al. Unipolar peak-negative voltage as an endocardial electrographic characteristic to predict overlying abnormal epicardial substrates in patients with right epicardial ventricular tachycardia. J Cardiovasc Electrophysiol. 2014; 25:1343–1349.
Article52. Jaïs P, Maury P, Khairy P, et al. Elimination of local abnormal ventricular activities: a new end point for substrate modification in patients with scar-related ventricular tachycardia. Circulation. 2012; 125:2184–2196.53. Silberbauer J, Oloriz T, Maccabelli G, et al. Noninducibility and late potential abolition: a novel combined prognostic procedural end point for catheter ablation of postinfarction ventricular tachycardia. Circ Arrhythm Electrophysiol. 2014; 7:424–435.54. Nogami A, Sugiyasu A, Tada H, et al. Changes in the isolated delayed component as an endpoint of catheter ablation in arrhythmogenic right ventricular cardiomyopathy: predictor for long-term success. J Cardiovasc Electrophysiol. 2008; 19:681–688.
Article55. Ellison KE, Friedman PL, Ganz LI, et al. Entrainment mapping and radiofrequency catheter ablation of ventricular tachycardia in right ventricular dysplasia. J Am Coll Cardiol. 1998; 32:724–728.
Article56. Tung R. Challenges and pitfalls of entrainment mapping of ventricular tachycardia: ten illustrative concepts. Circ Arrhythm Electrophysiol. 2017; 10:e004560.
Article57. Anter E, Tschabrunn CM, Buxton AE, et al. High-resolution mapping of postinfarction reentrant ventricular tachycardia: electrophysiological characterization of the circuit. Circulation. 2016; 134:314–327.58. Reithmann C, Hahnefeld A, Remp T, et al. Electroanatomic mapping of endocardial right ventricular activation as a guide for catheter ablation in patients with arrhythmogenic right ventricular dysplasia. Pacing Clin Electrophysiol. 2003; 26:1308–1316.
Article59. de Chillou C, Groben L, Magnin-Poull I, et al. Localizing the critical isthmus of postinfarct ventricular tachycardia: the value of pace-mapping during sinus rhythm. Heart Rhythm. 2014; 11:175–181.
Article60. Nayyar S, Wilson L, Ganesan AN, et al. High-density mapping of ventricular scar: a comparison of ventricular tachycardia (VT) supporting channels with channels that do not support VT. Circ Arrhythm Electrophysiol. 2014; 7:90–98.61. Di Biase L, Santangeli P, Burkhardt DJ, et al. Endo-epicardial homogenization of the scar versus limited substrate ablation for the treatment of electrical storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2012; 60:132–141.
Article62. Berruezo A, Fernández-Armenta J, Andreu D, et al. Scar dechanneling: new method for scar-related left ventricular tachycardia substrate ablation. Circ Arrhythm Electrophysiol. 2015; 8:326–336.63. Tzou WS, Frankel DS, Hegeman T, et al. Core isolation of critical arrhythmia elements for treatment of multiple scar-based ventricular tachycardias. Circ Arrhythm Electrophysiol. 2015; 8:353–361.
Article64. Campos B, Jauregui ME, Marchlinski FE, et al. Use of a novel fragmentation map to identify the substrate for ventricular tachycardia in postinfarction cardiomyopathy. Heart Rhythm. 2015; 12:95–103.
Article65. Jackson N, Gizurarson S, Viswanathan K, et al. Decrement evoked potential mapping: basis of a mechanistic strategy for ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. 2015; 8:1433–1442.66. Jamil-Copley S, Vergara P, Carbucicchio C, et al. Application of ripple mapping to visualize slow conduction channels within the infarct-related left ventricular scar. Circ Arrhythm Electrophysiol. 2015; 8:76–86.
Article67. Luther V, Linton NW, Jamil-Copley S, et al. A prospective study of ripple mapping the post-infarct ventricular scar to guide substrate ablation for ventricular tachycardia. Circ Arrhythm Electrophysiol. 2016; 9:e004072.
Article68. Irie T, Yu R, Bradfield JS, et al. Relationship between sinus rhythm late activation zones and critical sites for scar-related ventricular tachycardia: systematic analysis of isochronal late activation mapping. Circ Arrhythm Electrophysiol. 2015; 8:390–399.69. Aziz Z, Tung R. Novel mapping strategies for ventricular tachycardia ablation. Curr Treat Options Cardiovasc Med. 2018; 20:34.
Article70. d'Avila A, Houghtaling C, Gutierrez P, et al. Catheter ablation of ventricular epicardial tissue: a comparison of standard and cooled-tip radiofrequency energy. Circulation. 2004; 109:2363–2369.71. Sosa E, Scanavacca M, d'Avila A, et al. A new technique to perform epicardial mapping in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 1996; 7:531–536.
Article72. Maccabelli G, Tsiachris D, Silberbauer J, et al. Imaging and epicardial substrate ablation of ventricular tachycardia in patients late after myocarditis. Europace. 2014; 16:1363–1372.
Article73. Soejima K, Stevenson WG, Sapp JL, et al. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004; 43:1834–1842.74. Chung FP, Raharjo SB, Lin YJ, et al. A novel method to enhance phenotype, epicardial functional substrates, and ventricular tachyarrhythmias in Brugada syndrome. Heart Rhythm. 2017; 14:508–517.
Article75. Della Bella P, Brugada J, Zeppenfeld K, et al. Epicardial ablation for ventricular tachycardia: a European multicenter study. Circ Arrhythm Electrophysiol. 2011; 4:653–659.76. Sosa E, Scanavacca M, d'Avila A, et al. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J Am Coll Cardiol. 2000; 35:1442–1449.
Article77. Ozturk MT, Ebinç FA, Okyay GU, et al. Epicardial adiposity is associated with microalbuminuria in patients with essential hypertension. Acta Cardiol Sin. 2017; 33:74–80.78. Aydın E, Altın C, Sakallıoğlu O, et al. Epicardial adipose tissue thickness and carotid intima-media thickness in hemodialysis patients. Acta Cardiol Sin. 2017; 33:266–272.79. Ramazan Oncel C, Kucuk M. The value of epicardial adipose tissue thickness for cardiovascular risk stratification in hypertensive patients. Acta Cardiol Sin. 2017; 33:559.80. Sacher F, Roberts-Thomson K, Maury P, et al. Epicardial ventricular tachycardia ablation a multicenter safety study. J Am Coll Cardiol. 2010; 55:2366–2372.81. Desjardins B, Morady F, Bogun F. Effect of epicardial fat on electroanatomical mapping and epicardial catheter ablation. J Am Coll Cardiol. 2010; 56:1320–1327.
Article82. van Huls van Taxis CF, Wijnmaalen AP, Piers SR, et al. Real-time integration of MDCT-derived coronary anatomy and epicardial fat: impact on epicardial electroanatomic mapping and ablation for ventricular arrhythmias. JACC Cardiovasc Imaging. 2013; 6:42–52.83. Sosa E, Scanavacca M. Epicardial mapping and ablation techniques to control ventricular tachycardia. J Cardiovasc Electrophysiol. 2005; 16:449–452.
Article84. Bai R, Patel D, Di Biase L, et al. Phrenic nerve injury after catheter ablation: should we worry about this complication? J Cardiovasc Electrophysiol. 2006; 17:944–948.
Article85. Roberts-Thomson KC, Steven D, Seiler J, et al. Coronary artery injury due to catheter ablation in adults: presentations and outcomes. Circulation. 2009; 120:1465–1473.86. Dalal D, Jain R, Tandri H, et al. Long-term efficacy of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2007; 50:432–440.87. Verma A, Kilicaslan F, Schweikert RA, et al. Short- and long-term success of substrate-based mapping and ablation of ventricular tachycardia in arrhythmogenic right ventricular dysplasia. Circulation. 2005; 111:3209–3216.
Article88. Wei W, Liao H, Xue Y, et al. Long-term outcomes of radio-frequency catheter ablation on ventricular tachycardias due to arrhythmogenic right ventricular cardiomyopathy: a single center experience. PLoS One. 2017; 12:e0169863.
Article89. Satomi K, Kurita T, Suyama K, et al. Catheter ablation of stable and unstable ventricular tachycardias in patients with arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2006; 17:469–476.
Article90. Marchlinski FE, Callans DJ, Gottlieb CD, et al. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000; 101:1288–1296.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Catheter Ablation of Ventricular Tachycardia/Fibrillation in a Patient with Right Ventricular Amyloidosis with Initial Manifestations Mimicking Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy
- A Case of Arrythmogenic Right Ventricular Dysplasia
- Successful Treatment of Tachycardia-induced Cardiomyopathy with Radiofrequency Catheter Ablation
- A Case of Arrhythmogenic Right Ventricular Dysplasia
- Catheter Ablation of Ventricular Tachycardia in Patients with Post-Infarction Cardiomyopathy