Tuberc Respir Dis.
2018 Oct;81(4):299-304. 10.4046/trd.2018.0015.
Incidence of Adverse Effects and Discontinuation Rate between Patients Receiving 250 Micrograms and 500 Micrograms of Roflumilast: A Comparative Study
- Affiliations
-
- 1Division of Pulmonary, Allergy and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. chinkook77@gmail.com
- 2Division of Pulmonology and Critical Care Medicine, Department of Internal Medicine, Inje University Haeundae Paik Hospital, Inje University College of Medicine, Busan, Korea.
- KMID: 2420563
- DOI: http://doi.org/10.4046/trd.2018.0015
Abstract
- BACKGROUND
Roflumilast is the only approved oral phosphodiesterase-4 inhibitor for the treatment of severe chronic obstructive pulmonary disease (COPD) in patients with chronic bronchitis and a history of frequent exacerbations. The purpose of this study was to examine the incidence of adverse effects associated with roflumilast treatment in a real-world setting. Further, we compared the incidence of adverse effects and the discontinuation rate among patients receiving different doses.
METHODS
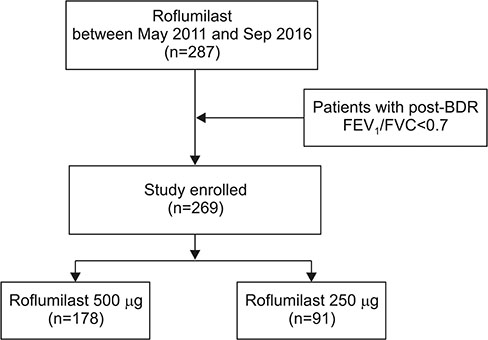
We identified all outpatients diagnosed with COPD at Seoul St. Mary's Hospital between May 2011 and September 2016 and retrospectively reviewed their medical records. Roflumilast was prescribed to patients in doses of 500 µg and 250 µg.
RESULTS
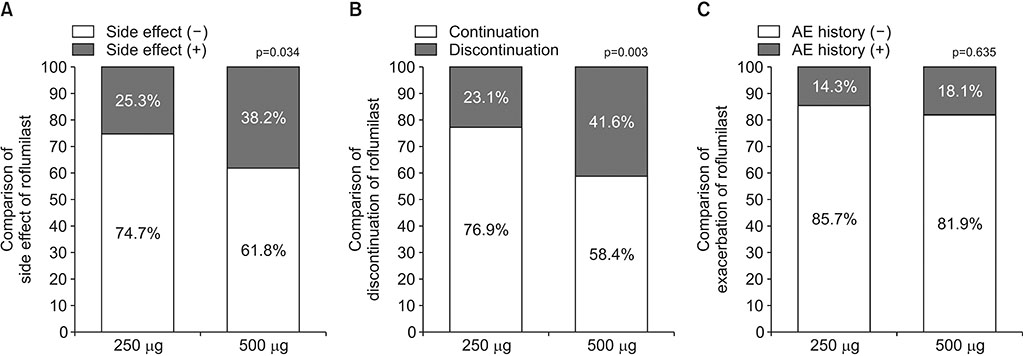
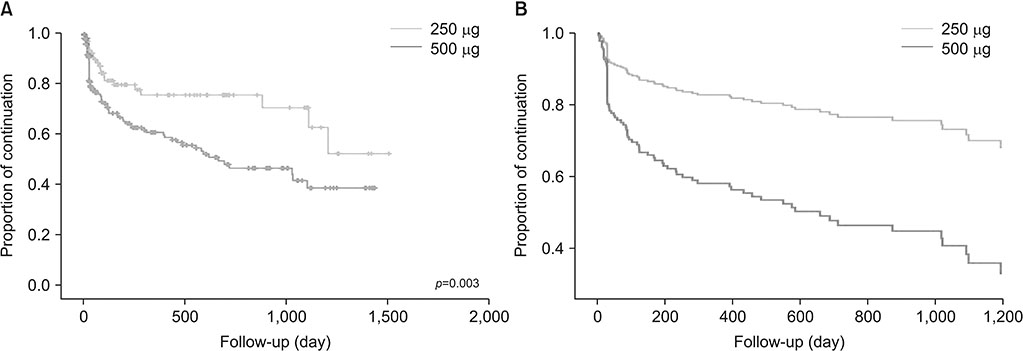
A total of 269 COPD patients were prescribed roflumilast in our hospital during the study period. Among them, 178 patients were treated with 500 µg and 91 patients were treated with 250 µg. The incidence of adverse effects was 38.2% in the 500 µg group and 25.3% in the 250 µg group (p=0.034). The discontinuation rate of roflumilast was 41.6% (n=74) in the 500 µg group and 23.1% (n=21) in the 250 µg group (p=0.003). When adjusted by age, sex, smoking status, and lung function, 500 µg dose was significantly associated with the discontinuation of roflumilast (odds ratio, 2.87; p < 0.001).
CONCLUSION
There was a lower incidence of adverse effects and discontinuation among patients treated with 250 µg compared with 500 µg dose. Further studies regarding the optimal dose of roflumilast are required.
MeSH Terms
Figure
Cited by 1 articles
-
Pharmacotherapy for chronic obstructive pulmonary disease
In Ae Kim, Yong Bum Park, Kwang Ha Yoo
J Korean Med Assoc. 2018;61(9):545-551. doi: 10.5124/jkma.2018.61.9.545.
Reference
-
1. Hatzelmann A, Morcillo EJ, Lungarella G, Adnot S, Sanjar S, Beume R, et al. The preclinical pharmacology of roflumilast: a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol Ther. 2010; 23:235–256.2. Rabe KF, Bateman ED, O'Donnell D, Witte S, Bredenbroker D, Bethke TD. Roflumilast: an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2005; 366:563–571.3. Calverley PM, Sanchez-Toril F, McIvor A, Teichmann P, Bredenbroeker D, Fabbri LM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007; 176:154–161.
Article4. Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ, et al. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009; 374:685–694.
Article5. Fabbri LM, Calverley PM, Izquierdo-Alonso JL, Bundschuh DS, Brose M, Martinez FJ, et al. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomised clinical trials. Lancet. 2009; 374:695–703.
Article6. Rennard SI, Calverley PM, Goehring UM, Bredenbroker D, Martinez FJ. Reduction of exacerbations by the PDE4 inhibitor roflumilast: the importance of defining different subsets of patients with COPD. Respir Res. 2011; 12:18.
Article7. Lee SD, Hui DS, Mahayiddin AA, Roa CC Jr, Kwa KH, Goehring UM, et al. Roflumilast in Asian patients with COPD: a randomized placebo-controlled trial. Respirology. 2011; 16:1249–1257.
Article8. O'Donnell DE, Bredenbroker D, Brose M, Webb KA. Physiological effects of roflumilast at rest and during exercise in COPD. Eur Respir J. 2012; 39:1104–1112.9. Zheng J, Yang J, Zhou X, Zhao L, Hui F, Wang H, et al. Roflumilast for the treatment of COPD in an Asian population: a randomized, double-blind, parallel-group study. Chest. 2014; 145:44–52.10. Michalski JM, Golden G, Ikari J, Rennard SI. PDE4: a novel target in the treatment of chronic obstructive pulmonary disease. Clin Pharmacol Ther. 2012; 91:134–142.
Article11. Munoz-Esquerre M, Diez-Ferrer M, Monton C, Pomares X, Lopez-Sanchez M, Huertas D, et al. Roflumilast added to triple therapy in patients with severe COPD: a real life study. Pulm Pharmacol Ther. 2015; 30:16–21.12. Hwang H, Shin JY, Park KR, Shin JO, Song KH, Park J, et al. Effect of a dose-escalation regimen for improving adherence to Roflumilast in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis. 2015; 78:321–325.
Article13. Rogliani P, Calzetta L, Cazzola M, Matera MG. Drug safety evaluation of roflumilast for the treatment of COPD: a meta-analysis. Expert Opin Drug Saf. 2016; 15:1133–1146.
Article14. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005; 26:319–338.15. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Arch Bronconeumol. 2017; 53:128–149.
Article16. Torphy TJ, Barnette MS, Underwood DC, Griswold DE, Christensen SB, Murdoch RD, et al. Ariflo (SB 207499), a second generation phosphodiesterase 4 inhibitor for the treatment of asthma and COPD: from concept to clinic. Pulm Pharmacol Ther. 1999; 12:131–135.17. Lahu G, Facius A. Application of population pharmacokinetic modeling to explore the impact of alternative roflumilast dosing regimens on tolerability. Int J Clin Pharmacol Ther. 2013; 51:832–836.
Article18. Park TS, Lee JS, Seo JB, Hong Y, Yoo JW, Kang BJ, et al. Study design and outcomes of Korean Obstructive Lung Disease (KOLD) Cohort Study. Tuberc Respir Dis. 2014; 76:169–174.
Article19. Lee JY, Chon GR, Rhee CK, Kim DK, Yoon HK, Lee JH, et al. Characteristics of patients with chronic obstructive pulmonary disease at the first visit to a pulmonary medical center in Korea: The KOrea COpd Subgroup Study Team Cohort. J Korean Med Sci. 2016; 31:553–560.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of a Dose-Escalation Regimen for Improving Adherence to Roflumilast in Patients with Chronic Obstructive Pulmonary Disease
- The inflammatory potential of surgical glove lubricants: Biosorb, Keoflo, calcium carbonate and Hydrocote after intravitreal injection
- Chemosensitization to adriamycin by cyclosporin A and verapamil in human retinoblastoma cell lines
- Estimation of the geometric means and the reference values of blood lead levels among Koreans
- Resolution of experimental intravitreal fibrin by tissue plasminogen activator