Transl Clin Pharmacol.
2018 Mar;26(1):10-15. 10.12793/tcp.2018.26.1.10.
Development of R packages: ‘NonCompart’ and ‘ncar’ for noncompartmental analysis (NCA)
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, University of Ulsan, Seoul 05505, Republic of Korea. ksbae@amc.seoul.kr
- KMID: 2420302
- DOI: http://doi.org/10.12793/tcp.2018.26.1.10
Abstract
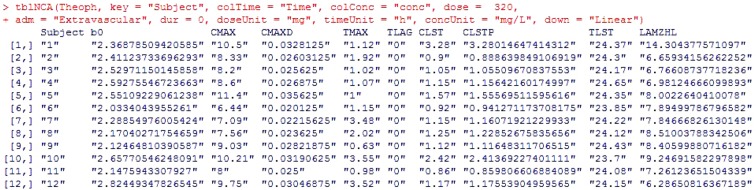
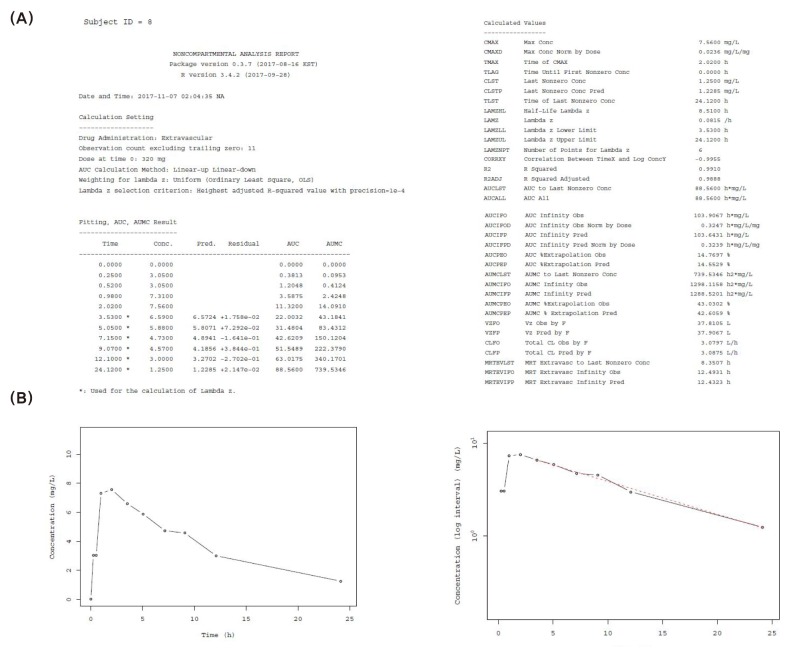
- Noncompartmental analysis (NCA) is a primary analytical approach for pharmacokinetic studies, and its parameters act as decision criteria in bioequivalent studies. Currently, NCA is usually carried out by commercial softwares such as WinNonlin®. In this article, we introduce our newly-developed two R packages, NonCompart (NonCompartmental analysis for pharmacokinetic data) and ncar (NonCompartmental Analysis for pharmacokinetic Report), which can perform NCA and produce complete NCA reports in both pdf and rtf formats. These packages are compatible with CDISC (Clinical Data Interchange Standards Consortium) standard as well. We demonstrate how the results of WinNonlin® are reproduced and how NCA reports can be obtained. With these R packages, we aimed to help researchers carry out NCA and utilize the output for early stages of drug development process. These R packages are freely available for download from the CRAN repository.
Keyword
Figure
Cited by 1 articles
-
An experience on the model-based evaluation of pharmacokinetic drug-drug interaction for a long half-life drug
Yunjung Hong, Sangil Jeon, Suein Choi, Sungpil Han, Maria Park, Seunghoon Han
Korean J Physiol Pharmacol. 2021;25(6):545-553. doi: 10.4196/kjpp.2021.25.6.545.
Reference
-
1. Acharya C, Hooker AC, Turkyilmaz GY, Jonsson S, Karlsson MO. A diagnostic tool for population models using non-compartmental analysis: The ncappc package for R. Comput Methods Programs Biomed. 2016; 127:83–93. DOI: 10.1016/j.cmpb.2016.01.013. PMID: 27000291.
Article2. Gabrielsson J, Weiner D. Non-compartmental analysis. Methods Mol Biol. 2012; 929:377. PMID: 23007438.
Article3. The R Project for Statistical Computing. R. Accessed 6 November 2017. http://www.r-project.org/.4. Certara. Phoenix WinNonlin®. Accessed 28 February 2018. https://www.certara.com/software/pkpd-modeling-and-simulation/phoenix-winnonlin/?ap%5B0%5D=PKPD/.5. Thermo Fisher Scientific, Kinetica. Accessed 28 February 2018. https://www.thermofisher.com/order/catalog/product/INF-24000/.6. Kim MG, Yim DS, Bae KS. R-based reproduction of the estimation process hidden behind NONMEN Part 1: first-order approximation method. Transl Clin Pharmacol. 2015; 23:1–7.7. Clinical Data Interchange Standards Consortium (CDISC). Study Data Tabulation Model Implementation Guide: Human Clinical Trials Version 3.2. Accessed 28 February 2018. https://www.cdisc.org/system/files/all/standard_category/application/pdf/sdtmig_v3.2.pdf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Development of R packages: ‘NonCompart’ and ‘ncar’ for noncompartmental analysis (NCA)
- Non-compartmental data analysis using SimBiology and MATLAB
- Changes of serum neutrophil chemotactic activity(NCA) and myeloperoxidase(MPO) level following lysine-aspirin(L-ASA) bronchoprovocation test in aspirin-sensitive asthmatic patients
- Development and Implementation of Problem-based Learning Packages on the Respiratory and Cardiac System
- Enhanced Serum Neutrophil Chemotactic Activity was Noted in Both Early and Late Asthmatic Responses During Lysine-Aspirin Bronchoprovocation Test in ASA-Sensitive Asthmatic Patients