Yonsei Med J.
2017 May;58(3):637-643. 10.3349/ymj.2017.58.3.637.
Efficacy and Safety of Different Aceclofenac Treatments for Chronic Lower Back Pain: Prospective, Randomized, Single Center, Open-Label Clinical Trials
- Affiliations
-
- 1Department of Orthopedic Surgery, Yonsei University College of Medicine, Seoul, Korea. shmoon@yuhs.ac
- 2Department of Orthopedic Surgery, Catholic-Kwandong University College of Medicine, International St. Mary's Hospital, Incheon, Korea.
- KMID: 2419124
- DOI: http://doi.org/10.3349/ymj.2017.58.3.637
Abstract
- PURPOSE
Nonsteroidal anti-inflammatory drugs are a mainstay for medical treatment of chronic lower back pain (CLBP). Increased dose intervals for medication have been associated with increased patient adherence to prescriptions. The purpose of this clinical trial was to compare the efficacy and safety of a once daily dose of aceclofenac controlled release (CR) and a twice daily dose of aceclofenac for CLBP management.
MATERIALS AND METHODS
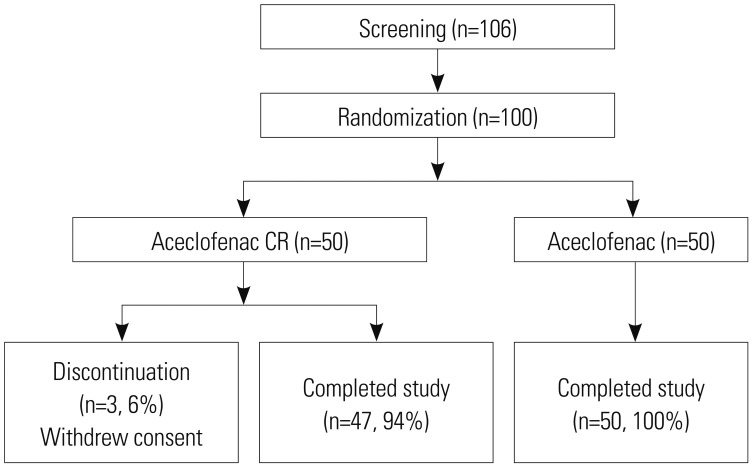
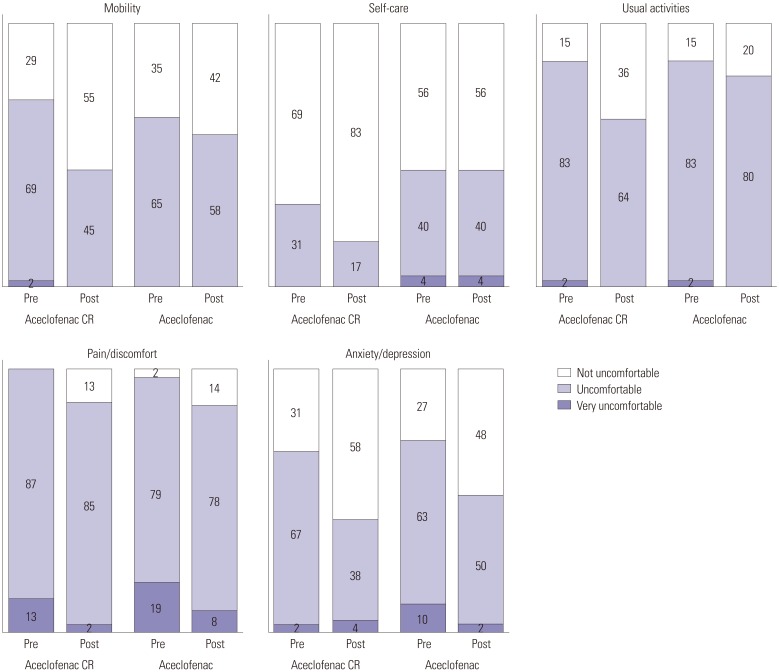
A prospective, randomized, single center, open-label clinical trial was performed to compare the efficacy and safety of aceclofenac CR (200 mg once daily) to aceclofenac dose (100 mg twice daily). Fifty patients in each group were enrolled for the study. The primary end point was Visual Analogue Scale (VAS) change at baseline to that at 2 weeks after medication and safety profiles. Also, change in quality of life measured by EuroQoL 5D (EQ-5D) and Oswestry Disability Index (ODI) functional score for the lumbar spine were also assessed.
RESULTS
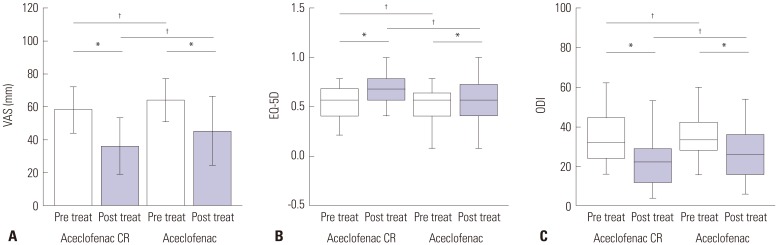
Within groups at pre- and post-treatment, there were significant VAS reductions for aceclofenac CR and aceclofenac (p=0.028). EQ-5D increased significantly in both groups (p=0.037). ODI scores decreased significantly in both groups (p=0.012). However, there were no significant differences between aceclofenac CR and aceclofenac at pre- and post-treatment. Patients with aceclofenac CR showed significant increases in heartburn and indigestion and adverse gastrointestinal effects, compared to aceclofenac.
CONCLUSION
In patients with CLBP, aceclofenac CR and aceclofenac demonstrated significant symptomatic pain relief, improvement in quality of life and functional scores. Aceclofenac CR slightly increased gastrointestinal adverse effects, such as heartburn and indigestion.
Keyword
MeSH Terms
-
Administration, Oral
Adult
Aged
Anti-Inflammatory Agents, Non-Steroidal/*administration & dosage/therapeutic use
Diclofenac/administration & dosage/*analogs & derivatives/therapeutic use
Drug Administration Schedule
Female
Humans
Low Back Pain/*drug therapy
Male
Middle Aged
Pain Measurement
Prospective Studies
Quality of Life
Treatment Outcome
Anti-Inflammatory Agents, Non-Steroidal
Diclofenac
Figure
Reference
-
1. Witt CM, Pach D, Reinhold T, Wruck K, Brinkhaus B, Mank S, et al. Treatment of the adverse effects from acupuncture and their economic impact: a prospective study in 73,406 patients with low back or neck pain. Eur J Pain. 2011; 15:193–197. PMID: 20609604.2. Andrade NS, Flynn JP, Bartanusz V. Twenty-year perspective of randomized controlled trials for surgery of chronic nonspecific low back pain: citation bias and tangential knowledge. Spine J. 2013; 13:1698–1704. PMID: 24012430.
Article3. Chaparro LE, Furlan AD, Deshpande A, Mailis-Gagnon A, Atlas S, Turk DC. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane Review. Spine (Phila Pa 1976). 2014; 39:556–563. PMID: 24480962.4. Cawston H, Davie A, Paget MA, Skljarevski V, Happich M. Efficacy of duloxetine versus alternative oral therapies: an indirect comparison of randomised clinical trials in chronic low back pain. Eur Spine J. 2013; 22:1996–2009. PMID: 23686477.
Article5. Schattenkirchner M, Milachowski KA. A double-blind, multicentre, randomised clinical trial comparing the efficacy and tolerability of aceclofenac with diclofenac resinate in patients with acute low back pain. Clin Rheumatol. 2003; 22:127–135. PMID: 12740678.
Article6. Li J, Mansmann UR. Modeling of non-steroidal anti-inflammatory drug effect within signaling pathways and miRNA-regulation pathways. PLoS One. 2013; 8:e72477. PMID: 23967306.
Article7. Day RO, Graham GG. Non-steroidal anti-inflammatory drugs (NSAIDs). BMJ. 2013; 346:f3195. PMID: 23757736.
Article8. Bae SK, Kim SH, Lee HW, Seong SJ, Shin SY, Lee SH, et al. Pharmacokinetics of a new once-daily controlled-release formulation of aceclofenac in Korean healthy subjects compared with immediate-release aceclofenac and the effect of food: a randomized, open-label, three-period, crossover, single-centre study. Clin Drug Investig. 2012; 32:111–119.9. Legrand E. Aceclofenac in the management of inflammatory pain. Expert Opin Pharmacother. 2004; 5:1347–1357. PMID: 15163279.
Article10. Pareek A, Chandurkar N, Chandanwale AS, Ambade R, Gupta A, Bartakke G. Aceclofenac-tizanidine in the treatment of acute low back pain: a double-blind, double-dummy, randomized, multicentric, comparative study against aceclofenac alone. Eur Spine J. 2009; 18:1836–1842. PMID: 19421791.
Article11. Trelle S, Reichenbach S, Wandel S, Hildebrand P, Tschannen B, Villiger PM, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011; 342:c7086. PMID: 21224324.
Article12. Vestergaard P, Hermann P, Jensen JE, Eiken P, Mosekilde L. Effects of paracetamol, non-steroidal anti-inflammatory drugs, acetylsalicylic acid, and opioids on bone mineral density and risk of fracture: results of the Danish Osteoporosis Prevention Study (DOPS). Osteoporos Int. 2012; 23:1255–1265. PMID: 21710339.
Article13. Pareek A, Chandurkar N, Gupta A, Sirsikar A, Dalal B, Jesalpura B, et al. Efficacy and safety of aceclofenac-cr and aceclofenac in the treatment of knee osteoarthritis: a 6-week, comparative, randomized, multicentric, double-blind study. J Pain. 2011; 12:546–553. PMID: 21277837.
Article14. Ogon M, Krismer M, Söllner W, Kantner-Rumplmair W, Lampe A. Chronic low back pain measurement with Visual Analogue Scales in different settings. Pain. 1996; 64:425–428. PMID: 8783305.
Article15. Johnsen LG, Hellum C, Nygaard OP, Storheim K, Brox JI, Rossvoll , et al. Comparison of the SF6D, the EQ5D, and the Oswestry Disability Index in patients with chronic low back pain and degenerative disc disease. BMC Musculoskelet Disord. 2013; 14:148. PMID: 23622053.
Article16. Whynes DK, McCahon RA, Ravenscroft A, Hodgkinson V, Evley R, Hardman JG. Responsiveness of the EQ-5D health-related quality-of-life instrument in assessing low back pain. Value Health. 2013; 16:124–132. PMID: 23337223.
Article17. Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980; 66:271–273. PMID: 6450426.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Multi-center, Double-blind, Randomized and Comparative Clinical Study for the Safety and Analgesic Effect after four-week-treatment with Neurotropin(R) in Patients with Low Back Pain: Compared to Aceclofenac
- Comparison of pharmacokinetics and safety of fixed-dose combination of SKI306X and aceclofenac versus separate tablets in healthy subjects
- Effects of JOINS® on the pharmacokinetic profiles of aceclofenac in healthy Korean volunteers: an open-label, multiple-dose, one sequence, two-period study
- Analgesic Effects of Aceclofenac in Adult Post Tonsillectomy Pain
- Comparative efficacy of bromelain and aceclofenac in limiting post-operative inflammatory sequelae in surgical removal of lower impacted third molar: a randomized controlled, triple blind clinical trial