Yonsei Med J.
2017 Sep;58(5):981-987. 10.3349/ymj.2017.58.5.981.
Localization of Oncogenic Osteomalacia by Systemic Venous Sampling of Fibroblast Growth Factor 23
- Affiliations
-
- 1Department of Internal Medicine, Endocrine Research Institute, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. yumie@yuhs.ac
- 2Imperial College London, London, United Kingdom.
- 3Department of Radiology, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2418934
- DOI: http://doi.org/10.3349/ymj.2017.58.5.981
Abstract
- PURPOSE
Tumor-induced osteomalacia (TIO) is characterized by hypophosphatemia caused by a phosphaturic mesenchymal tumor. While surgical resection of the tumor leads to a cure, identification of the responsible tumor is challenging. Recently, several studies showed that systemic sampling of fibroblast growth factor 23 (FGF23) is helpful for localization of tumors. The present study aimed to evaluate the clinical utility of this method in Korean patients.
MATERIALS AND METHODS
Six patients compatible with TIO who were admitted to our hospital between 2006 and 2015 were analyzed. Systemic venous sampling of FGF23 was performed to detect blind lesions or to confirm a suspicious lesion identified in previous imaging studies.
RESULTS
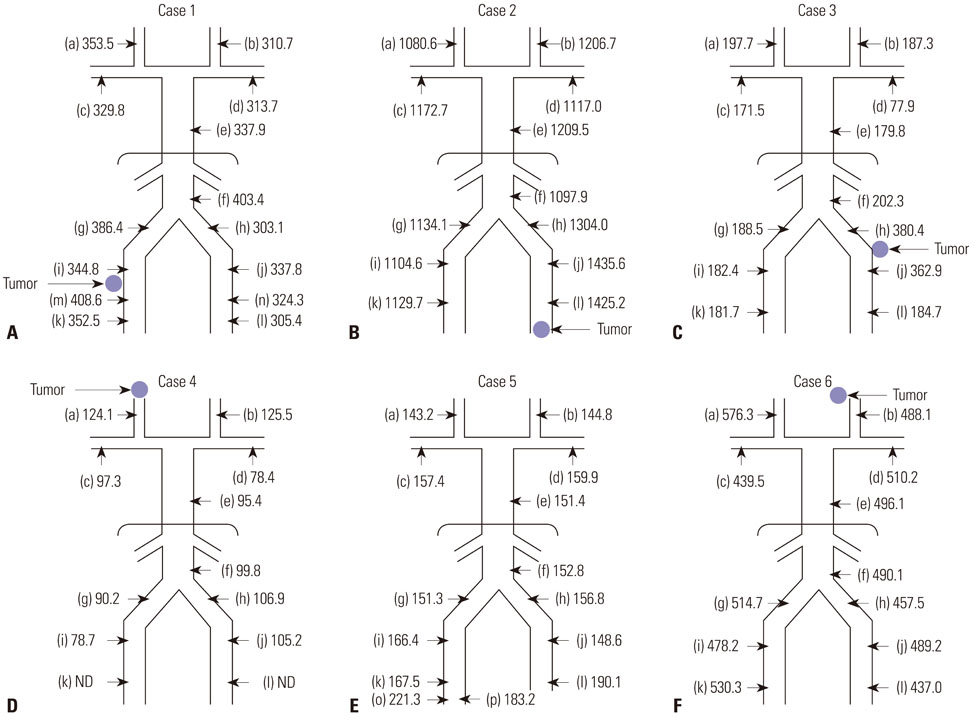
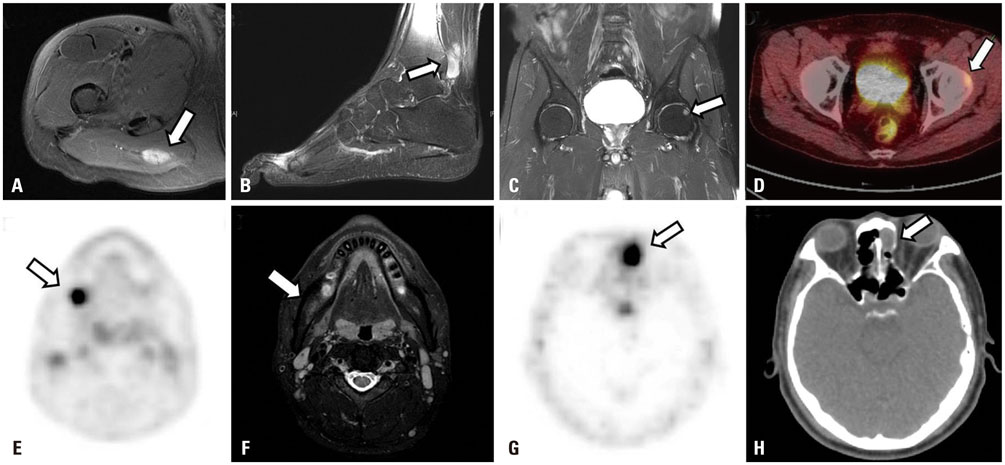
Venous sampling helped confirming the tumor in five of the six patients. Three patients underwent surgery after sampling, and in two patients, the lesions were detected after 3 years by means of 68Ga-DOTATOC positron emission tomography with computed tomography. In one patient, there was a local elevation of serum FGF23 without any related lesion on additional imaging.
CONCLUSION
Our data strengthened the value of venous sampling of FGF23 in predicting the location of tumors and suggested that it can be more effective in the presence of the relevant lesion in subsequent imaging analyses.
MeSH Terms
-
Adolescent
Adult
Fibroblast Growth Factors/*blood
Humans
Magnetic Resonance Imaging
Male
Middle Aged
Neoplasms, Connective Tissue/*blood/diagnostic imaging/*pathology
Octreotide/analogs & derivatives/chemistry
Organometallic Compounds/chemistry
Veins/*pathology
Young Adult
Organometallic Compounds
Fibroblast Growth Factors
Octreotide
Figure
Reference
-
1. Chong WH, Molinolo AA, Chen CC, Collins MT. Tumor-induced osteomalacia. Endocr Relat Cancer. 2011; 18:R53–R77.
Article2. Hautmann AH, Hautmann MG, Kölbl O, Herr W, Fleck M. Tumor-induced osteomalacia: an up-to-date review. Curr Rheumatol Rep. 2015; 17:512.
Article3. Fukumoto S. Diagnostic modalities for FGF23-producing tumors in patients with tumor-induced osteomalacia. Endocrinol Metab (Seoul). 2014; 29:136–143.
Article4. Shimada T, Urakawa I, Yamazaki Y, Hasegawa H, Hino R, Yoneya T, et al. FGF-23 transgenic mice demonstrate hypophosphatemic rickets with reduced expression of sodium phosphate cotransporter type IIa. Biochem Biophys Res Commun. 2004; 314:409–414.
Article5. Ito N, Shimizu Y, Suzuki H, Saito T, Okamoto T, Hori M, et al. Clinical utility of systemic venous sampling of FGF23 for identifying tumours responsible for tumour-induced osteomalacia. J Intern Med. 2010; 268:390–394.
Article6. Takeuchi Y, Suzuki H, Ogura S, Imai R, Yamazaki Y, Yamashita T, et al. Venous sampling for fibroblast growth factor-23 confirms preoperative diagnosis of tumor-induced osteomalacia. J Clin Endocrinol Metab. 2004; 89:3979–3982.
Article7. Nasu T, Kurisu S, Matsuno S, Tatsumi K, Kakimoto T, Kobayashi M, et al. Tumor-induced hypophosphatemic osteomalacia diagnosed by the combinatory procedures of magnetic resonance imaging and venous sampling for FGF23. Intern Med. 2008; 47:957–961.
Article8. van Boekel G, Ruinemans-Koerts J, Joosten F, Dijkhuizen P, van Sorge A, de Boer H. Tumor producing fibroblast growth factor 23 localized by two-staged venous sampling. Eur J Endocrinol. 2008; 158:431–437.
Article9. Westerberg PA, Olauson H, Toss G, Wikström B, Morales O, Linde T, et al. Preoperative tumor localization by means of venous sampling for fibroblast growth factor-23 in a patient with tumor-induced osteomalacia. Endocr Pract. 2008; 14:362–367.
Article10. Andreopoulou P, Dumitrescu CE, Kelly MH, Brillante BA, Cutler Peck CM, Wodajo FM, et al. Selective venous catheterization for the localization of phosphaturic mesenchymal tumors. J Bone Miner Res. 2011; 26:1295–1302.
Article11. Folpe AL, Fanburg-Smith JC, Billings SD, Bisceglia M, Bertoni F, Cho JY, et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol. 2004; 28:1–30.
Article12. McCance RA. Osteomalacia with Looser's nodes (Milkman's syndrome) due to a raised resistance to vitamin D acquired about the age of 15 years. Q J Med. 1947; 16:33–46.13. Prader A, Illig R, Uehlinger E, Stalder G. [Rickets following bone tumor]. Helv Paediatr Acta. 1959; 14:554–565.14. Jan de Beur SM. Tumor-induced osteomalacia. JAMA. 2005; 294:1260–1267.
Article15. Jan de Beur SM, Streeten EA, Civelek AC, McCarthy EF, Uribe L, Marx SJ, et al. Localisation of mesenchymal tumours by somatostatin receptor imaging. Lancet. 2002; 359:761–763.
Article16. Park YK. Oncogenic osteomalacia. Korean J Pathol. 2006; 40:1–8.17. Reubi JC, Waser B, Laissue JA, Gebbers JO. Somatostatin and vasoactive intestinal peptide receptors in human mesenchymal tumors: in vitro identification. Cancer Res. 1996; 56:1922–1931.18. Jiang Y, Xia WB, Xing XP, Silva BC, Li M, Wang O, et al. Tumor-induced osteomalacia: an important cause of adult-onset hypophosphatemic osteomalacia in China: report of 39 cases and review of the literature. J Bone Miner Res. 2012; 27:1967–1975.
Article19. Dupond JL, Mahammedi H, Prié D, Collin F, Gil H, Blagosklonov O, et al. Oncogenic osteomalacia: diagnostic importance of fibroblast growth factor 23 and F-18 fluorodeoxyglucose PET/CT scan for the diagnosis and follow-up in one case. Bone. 2005; 36:375–378.
Article20. Jagtap VS, Sarathi V, Lila AR, Malhotra G, Sankhe SS, Bandgar T, et al. Tumor-induced osteomalacia: a single center experience. Endocr Pract. 2011; 17:177–184.
Article21. Agrawal K, Bhadada S, Mittal BR, Shukla J, Sood A, Bhattacharya A, et al. Comparison of 18F-FDG and 68Ga DOTATATE PET/CT in localization of tumor causing oncogenic osteomalacia. Clin Nucl Med. 2015; 40:e6–e10.
Article22. Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A. 2001; 98:6500–6505.
Article23. Schiavi SC, Kumar R. The phosphatonin pathway: new insights in phosphate homeostasis. Kidney Int. 2004; 65:1–14.
Article24. Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003; 348:1656–1663.
Article25. Clifton-Bligh RJ, Hofman MS, Duncan E, Sim IeW, Darnell D, Clarkson A, et al. Improving diagnosis of tumor-induced osteomalacia with Gallium-68 DOTATATE PET/CT. J Clin Endocrinol Metab. 2013; 98:687–694.
Article26. Breer S, Brunkhorst T, Beil FT, Peldschus K, Heiland M, Klutmann S, et al. 68Ga DOTA-TATE PET/CT allows tumor localization in patients with tumor-induced osteomalacia but negative 111In-octreotide SPECT/CT. Bone. 2014; 64:222–227.
Article27. Antunes P, Ginj M, Zhang H, Waser B, Baum RP, Reubi JC, et al. Are radiogallium-labelled DOTA-conjugated somatostatin analogues superior to those labelled with other radiometals? Eur J Nucl Med Mol Imaging. 2007; 34:982–993.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A FGF23-Positive Maxillary Sinus Tumor Associated with Oncogenic Osteomalacia
- Diagnostic Modalities for FGF23-Producing Tumors in Patients with Tumor-Induced Osteomalacia
- Tumor-induced osteomalacia
- Ga68-DOTA Peptide PET/CT to Detect Occult Mesenchymal Tumor-Inducing Osteomalacia: A Case Series of Three Patients
- Acquired Forms of Fibroblast Growth Factor 23-Related Hypophosphatemic Osteomalacia