Yonsei Med J.
2017 Nov;58(6):1229-1236. 10.3349/ymj.2017.58.6.1229.
A Personalized and Learning Approach for Identifying Drugs with Adverse Events
- Affiliations
-
- 1Department of Internal Medicine, Nephrology Division, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 2Department of Surgery, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 3Department of Pharmacy, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 4FirstDIS Ltd., Seoul, Korea.
- 5Division of Business Administration, Sookmyung Women's University, Seoul, Korea. yokwon@sm.ac.kr
- 6Department of Urology, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 7Department of Internal Medicine, Nephrology Division, College of Medicine, Yonsei University, Seoul, Korea.
- KMID: 2418907
- DOI: http://doi.org/10.3349/ymj.2017.58.6.1229
Abstract
- PURPOSE
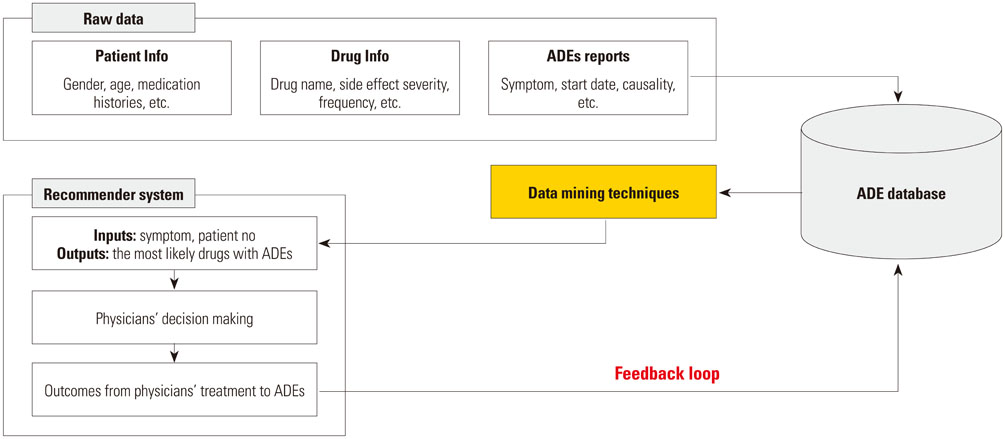
Adverse drug events (ADEs) are associated with high health and financial costs and have increased as more elderly patients treated with multiple medications emerge in an aging society. It has thus become challenging for physicians to identify drugs causing adverse events. This study proposes a novel approach that can improve clinical decision making with recommendations on ADE causative drugs based on patient information, drug information, and previous ADE cases.
MATERIALS AND METHODS
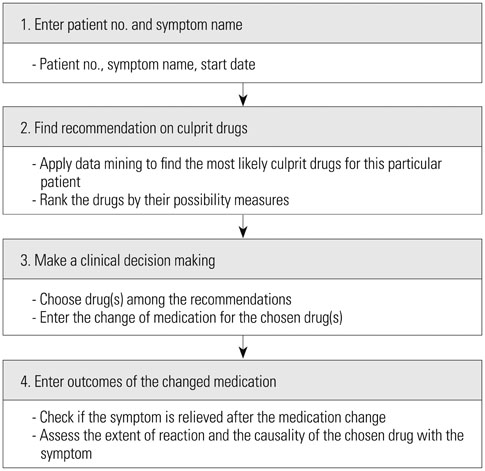
We introduce a personalized and learning approach for detecting drugs with a specific adverse event, where recommendations tailored to each patient are generated using data mining techniques. Recommendations could be improved by learning the associations of patients and ADEs as more ADE cases are accumulated through iterations. After consulting the system-generated recommendations, a physician can alter prescriptions accordingly and report feedback, enabling the system to evolve with actual causal relationships.
RESULTS
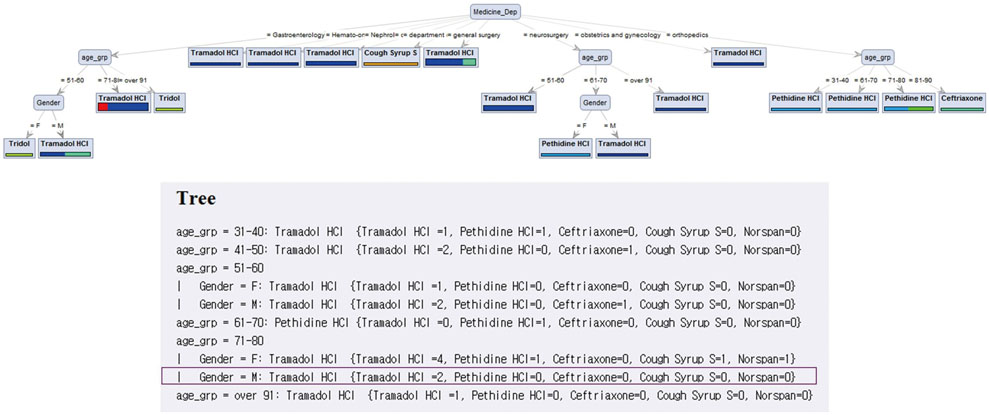
A prototype system is developed using ADE cases reported over 1.5 years and recommendations obtained from decision tree analysis are validated by physicians. Two representative cases demonstrate that the personalized recommendations could contribute to more prompt and accurate responses to ADEs.
CONCLUSION
The current system where the information of individual drugs exists but is not organized in such a way that facilitates the extraction of relevant information together can be complemented with the proposed approach to enhance the treatment of patients with ADEs. Our illustrative results show the promise of the proposed system and further studies are expected to validate its performance with quantitative measures.
MeSH Terms
Figure
Reference
-
1. Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. BMJ. 2004; 329:15–19.
Article2. Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006; 296:1858–1866.
Article3. Page RL 2nd, Ruscin JM. The risk of adverse drug events and hospital-related morbidity and mortality among older adults with potentially inappropriate medication use. Am J Geriatr Pharmacother. 2006; 4:297–305.
Article4. Sarkar U, López A, Maselli JH, Gonzales R. Adverse drug events in U.S. adult ambulatory medical care. Health Serv Res. 2011; 46:1517–1533.
Article5. Poudel DR, Acharya P, Ghimire S, Dhital R, Bharati R. Burden of hospitalizations related to adverse drug events in the USA: a retrospective analysis from large inpatient database. Pharmacoepidemiol Drug Saf. 2017; 26:635–641.
Article6. Leape LL, Bates DW, Cullen DJ, Cooper J, Demonaco HJ, Gallivan T, et al. Systems analysis of adverse drug events. JAMA. 1995; 274:35–43.
Article7. Food and Drug Administration. Safety Reporting Requirements for INDs and BA/BE Studies. 2012. https://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm227351.pdf.8. Bates DW, Boyle DL, Vander Vliet MB, Schneider J, Leape L. Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995; 10:199–205.
Article9. Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Ann Intern Med. 2004; 140:795–801.
Article10. Bourgeois FT, Shannon MW, Valim C, Mandl KD. Adverse drug events in the outpatient setting: an 11-year national analysis. Pharmacoepidemiol Drug Saf. 2010; 19:901–910.
Article11. Howard RL, Avery AJ, Howard PD, Partridge M. Investigation into the reasons for preventable drug related admissions to a medical admissions unit: observational study. Qual Saf Health Care. 2003; 12:280–285.
Article12. Kongkaew C, Hann M, Mandal J, Williams SD, Metcalfe D, Noyce PR, et al. Risk factors for hospital admissions associated with adverse drug events. Pharmacotherapy. 2013; 33:827–837.
Article13. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998; 279:1200–1205.
Article14. Bates DW, Spell N, Cullen DJ, Burdick E, Laird N, Petersen LA, et al. The costs of adverse drug events in hospitalized patients. JAMA. 1997; 277:307–311.
Article15. Ernst FR, Grizzle AJ. Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash). 2001; 41:192–199.
Article16. Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. JAMA. 1995; 274:29–34.
Article17. Jha AK, Kuperman GJ, Rittenberg E, Teich JM, Bates DW. Identifying hospital admissions due to adverse drug events using a computer-based monitor. Pharmacoepidemiol Drug Saf. 2001; 10:113–119.
Article18. Murff HJ, Patel VL, Hripcsak G, Bates DW. Detecting adverse events for patient safety research: a review of current methodologies. J Biomed Inform. 2003; 36:131–143.
Article19. Beuscart R, McNair P, Darmoni SJ, Koutkia V, Maglaveras N, Beuscart-Zephir MC, et al. Patient safety: detection and prevention of adverse drug events. Stud Health Technol Inform. 2009; 150:968–971.20. Lee JH, Park KH, Moon HJ, Lee YW, Park JW, Hong CS. Spontaneous reporting of adverse drug reactions through electronic submission from regional society healthcare professionals in Korea. Yonsei Med J. 2012; 53:1022–1027.
Article21. Park K, Soukavong M, Kim J, Kwon KE, Jin XM, Lee J, et al. Signal detection of imipenem compared to cther drugs from Korea adverse event reporting system database. Yonsei Med J. 2017; 58:564–569.
Article22. Chazard E, Ficheur G, Bernonville S, Luyckx M, Beuscart R. Data mining to generate adverse drug events detection rules. IEEE Trans Inf Technol Biomed. 2011; 15:823–830.
Article23. Chazard E, Merlin B, Ficheur G, Sarfati JC, Beuscart R. PSIP Consortium. Detection of adverse drug events: proposal of a data model. Stud Health Technol Inform. 2009; 148:63–74.24. Forster AJ, Jennings A, Chow C, Leeder C, van Walraven C. A systematic review to evaluate the accuracy of electronic adverse drug event detection. J Am Med Inform Assoc. 2012; 19:31–38.
Article25. Groves P, Kayyali B, Knott D, Van Kuiken S. The ‘big data’ revolution in healthcare. McKinsey Quarterly;2013.26. Leaman R, Wojtulewicz L, Sullivan R, Skariah A, Yang J, Gonzalez G. Towards internet-age pharmacovigilance: extracting adverse drug reactions from user posts to health-related social networks. In : Proc. 2010 Works BioNLP; 2010. p. 117–125.27. Benton A, Ungar L, Hill S, Hennessy S, Mao J, Chung A, et al. Identifying potential adverse effects using the web: a new approach to medical hypothesis generation. J Biomed Inform. 2011; 44:989–996.
Article28. Harpaz R, Vilar S, Dumouchel W, Salmasian H, Haerian K, Shah NH, et al. Combing signals from spontaneous reports and electronic health records for detection of adverse drug reactions. J Am Med Inform Assoc. 2013; 20:413–419.
Article29. Adomavicius G, Tuzhilin A. Toward the next generation of recommender systems: a survey of the state-of-the-art and possible extensions. IEEE Trans Knowl Data Eng. 2005; 17:734–749.
Article30. Linoff GS, Berry MJ. Data mining techniques: for marketing, sales, and customer relationship management. 3rd ed. John Wiley & Sons;2011. p. 888.31. Provost F, Fawcett T. Data science for business: what you need to know about data mining and data-analytic thinking. 1st ed. Sebastopol CA: O'Reilly Media;2013.32. Kotu V, Deshpande B. Predictive analytics and data mining: concepts and practice with rapidminer. Waltham MA: Morgan Kaufmann;2014.33. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000; 356:1255–1259.
Article34. Son MK, Lee YW, Jung HY, Yi SW, Lee KH, Kim SU, et al. Comparison of the Naranjo and WHO-Uppsala Monitoring Centre criteria for causality assessment of adverse drug reactions. Korean J Med. 2008; 74:181–187.35. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30:239–245.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Occurrence and Management of Adverse Skin Events due to Continuous Glucose Monitoring
- Adverse Effects of Antiepileptic Drugs
- Analysis of Important Medical Adverse Events and Signals Related with Cyclosporine and Tacrolimus Using the FDA Adverse Event Reporting System (FAERS) Database
- Comparative Analysis of Ethical-the-counter Drugs and Over-the-counter Drugs for the Adverse Events from the Community Pharmacy
- Pharmacogenomics in Drug Discovery and Development