Obstet Gynecol Sci.
2017 Nov;60(6):535-541. 10.5468/ogs.2017.60.6.535.
Efficacy and safety of venous thromboembolism prophylaxis with fondaparinux in women at risk after cesarean section
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Nara Medical University, Nara, Japan. kawaryu@naramed-u.ac.jp
- KMID: 2418354
- DOI: http://doi.org/10.5468/ogs.2017.60.6.535
Abstract
OBJECTIVES
Cesarean section is associated with an increased risk for venous thromboembolism (VTE). The safety and efficacy of primary prophylaxis of fondaparinux, a synthetic sulfated pentasaccharide heparin analog, in women at risk after cesarean section is uncertain.
METHODS
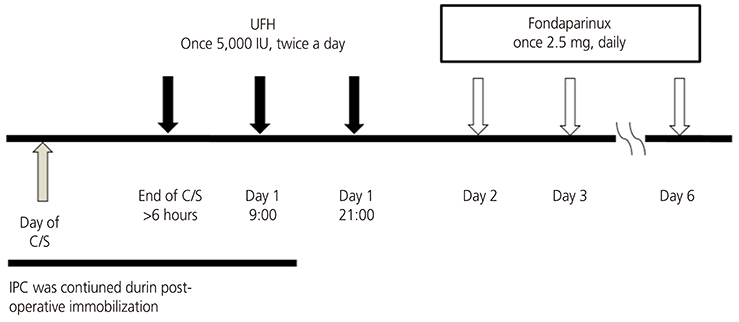
This was a retrospective study of 295 cases of pregnant women presenting to a tertiary referral center of Nara, Japan, to evaluate the usefulness of thromboprophylaxis with fondaparinux after cesarean delivery between 2011 and 2012. Patients were initially received unfractionated heparin (once 5,000 IU subcutaneously, twice a day), starting 6 hours after cesarean section for 24 hours, and then treated with fondaparinux (once 2.5 mg daily, subcutaneously) for 5 days. The primary efficacy end-point was an improvement in the incidence of symptomatic VTE or fatal post-cesarean pulmonary thromboembolism. The primary safety end-point was major bleeding during treatment.
RESULTS
There were neither any episodes of symptomatic VTE cases nor maternal deaths. A total of 10 patients had a bleeding event. Major bleeding complication was observed in 2 (0.68%) of 295 patients receiving fondaparinux. Non-major bleeding into critical sites was observed in 8 patients, often at surgical sites, and recovery was not delayed.
CONCLUSION
This study demonstrates the safety and efficacy of fondaparinux in women at high risk of VTE after cesarean section. Large phase trials comparing clinical outcomes with fondaparinux across a wide spectrum of patients are needed to confirm these observations.
MeSH Terms
Figure
Cited by 1 articles
-
Knowledge, Awareness and Risk of Occurrence of Venous Thromboembolism of Perinatal Women
Eun Sook Kim, Hye Young Kim
Korean J Women Health Nurs. 2019;25(2):154-168. doi: 10.4069/kjwhn.2019.25.2.154.
Reference
-
1. Anderson FA Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003; 107:I9–I16.2. Lidegaard Ø, Edström B, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception. 2002; 65:187–196.3. Donnelly JC, D’Alton ME. Pulmonary embolus in pregnancy. Semin Perinatol. 2013; 37:225–233.4. Sia WW, Powrie RO, Cooper AB, Larson L, Phipps M, Spencer P, et al. The incidence of deep vein thrombosis in women undergoing cesarean delivery. Thromb Res. 2009; 123:550–555.5. Giannubilo SR, Tranquilli AL. Anticoagulant therapy during pregnancy for maternal and fetal acquired and inherited thrombophilia. Curr Med Chem. 2012; 19:4562–4571.6. Bain E, Wilson A, Tooher R, Gates S, Davis LJ, Middleton P. Prophylaxis for venous thromboembolic disease in pregnancy and the early postnatal period. Cochrane Database Syst Rev. 2014; CD001689.7. Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-based Clinical Practice Guidelines. Chest. 2008; 133:844S–86S.8. Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-based Clinical Practice Guidelines. Chest. 2012; 141:e691S–e736S.9. Cohen AT. Asia-Pacific Thrombosis Advisory Board. Asia-Pacific Thrombosis Advisory Board consensus paper on prevention of venous thromboembolism after major orthopaedic surgery. Thromb Haemost. 2010; 104:919–930.10. Hasegawa M, Wada H, Wakabayashi H, Yoshida K, Miyamoto N, Asanuma K, et al. The relationships among hemostatic markers, the withdrawal of fondaparinux due to a reduction in hemoglobin and deep vein thrombosis in Japanese patients undergoing major orthopedic surgery. Clin Chim Acta. 2013; 425:109–113.11. Hosaka K, Saito S, Ishii T, Sumino T, Ryu K, Suzuki G, et al. Safety of fondaparinux versus enoxaparin after TKA in Japanese patients. Orthopedics. 2013; 36:e428–33.12. James AH. Venous thromboembolism in pregnancy. Arterioscler Thromb Vasc Biol. 2009; 29:326–331.13. Eikelboom JW, Karthikeyan G, Fagel N, Hirsh J. American Association of Orthopedic Surgeons and American College of Chest Physicians guidelines for venous thromboembolism prevention in hip and knee arthroplasty differ: what are the implications for clinicians and patients? Chest. 2009; 135:513–520.14. James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol. 2006; 194:1311–1315.15. Cavazza S, Rainaldi MP, Adduci A, Palareti G. Thromboprophylaxis following cesarean delivery: one site prospective pilot study to evaluate the application of a risk score model. Thromb Res. 2012; 129:28–31.16. Gader AA, Haggaz AE, Adam I. Epidemiology of deep venous thrombosis during pregnancy and puerperium in Sudanese women. Vasc Health Risk Manag. 2009; 5:85–87.17. Zhi-jian S, Yu Z, Giu-xing Q, Yi-peng W, Xi-sheng W, Hong Z, et al. Efficacy and safety of low molecular weight heparin prophylaxis for venous thromboembolism following lumbar decompression surgery. Chin Med Sci J. 2011; 26:221–226.18. Carlson MK, Gleason PP, Sen S. Elevation of hepatic transaminases after enoxaparin use: case report and review of unfractionated and low-molecular-weight heparin-induced hepatotoxicity. Pharmacotherapy. 2001; 21:108–113.19. Schünemann HJ, Cook D, Guyatt G. Methodology for antithrombotic and thrombolytic therapy guideline development: American College of Chest Physicians Evidence-based Clinical Practice Guidelines (8th Edition). Chest. 2008; 133:113S–122S.20. Ansell J, Hirsh J, Hylek E, Jacobson A, Crowther M, Palareti G. Pharmacology and management of the vitamin K antagonists: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008; 133:160S–198S.21. Johnson PN, Benefield EC, Bui PY, Marlar RA, Gessouroun MR. Fondaparinux in an obese child with heparin-induced thrombocytopenia and a history of renal failure. J Pediatr Pharmacol Ther. 2013; 18:303–310.22. Ciurzyński M, Jankowski K, Pietrzak B, Mazanowska N, Rzewuska E, Kowalik R, et al. Use of fondaparinux in a pregnant woman with pulmonary embolism and heparin-induced thrombocytopenia. Med Sci Monit. 2011; 17:CS56–CS59.23. Parody R, Oliver A, Souto JC, Fontcuberta J. Fondaparinux (ARIXTRA) as an alternative anti-thrombotic prophylaxis when there is hypersensitivity to low molecular weight and unfractionated heparins. Haematologica. 2003; 88:ECR32.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Verification of selective and individual pulmonary thromboembolism prophylaxes for cesarean delivery
- Knowledge, Awareness and Risk of Occurrence of Venous Thromboembolism of Perinatal Women
- Thromboembolism in pregnancy
- Two Cases of Pulmonary Thromboembolism and Deep Vein Thrombosis Developed After Cesarean Section
- Acute Pulmonary Embolism due to Thrombus-in-Transit in the Right Atrium During Bipolar Endoprosthesis of the Hip