Cancer Res Treat.
2018 Jul;50(3):791-800. 10.4143/crt.2017.044.
Chemotherapy versus Best Supportive Care in Advanced Biliary Tract Carcinoma: A Multi-institutional Propensity Score Matching Analysis
- Affiliations
-
- 1Division of Hematology-Oncology, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 2Division of Hematology and Oncology, Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- 3Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea.
- 4Regional Cardiocerebrovascular Center, Gyeongsang National University Hospital, Jinju, Korea.
- 5Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea.
- 7Department of Internal Medicine, Chung-Ang University College of Medicine, Seoul, Korea.
- 8Department of Internal Medicine, Gyeongsang National University School of Medicine, Jinju, Korea. newatp@naver.com
- KMID: 2417868
- DOI: http://doi.org/10.4143/crt.2017.044
Abstract
- PURPOSE
Although chemotherapy is recommended by various guidelines for advanced biliary tract cancer (BTC), the evidence supporting its use over best supportive care (BSC) is limited. The aim of this study was to investigate the survival benefit of chemotherapy over that of BSC in advanced BTC patients.
MATERIALS AND METHODS
Advanced BTC patientswith a good performance status (Eastern CooperativeOncologyGroup [ECOG] 0-2) were eligible for the study. Data were retrospectively collected from four tertiary cancer centers and analyzed using propensity score matching (PSM). Of the 604 patients enrolled, 206 received BSC and 398 received chemotherapy. PSM analysis was performed using the following variables: age, ECOG status, carcinoembryonic antigen (CEA) level, white blood cell level, albumin level, total bilirubin level, and aspartate aminotransferase level. The sample size of each group was 164 patients after PSM. Median survival was compared between the two groups by using the Kaplan-Meier method, and prognostic factors were investigated using Cox proportional regression analysis.
RESULTS
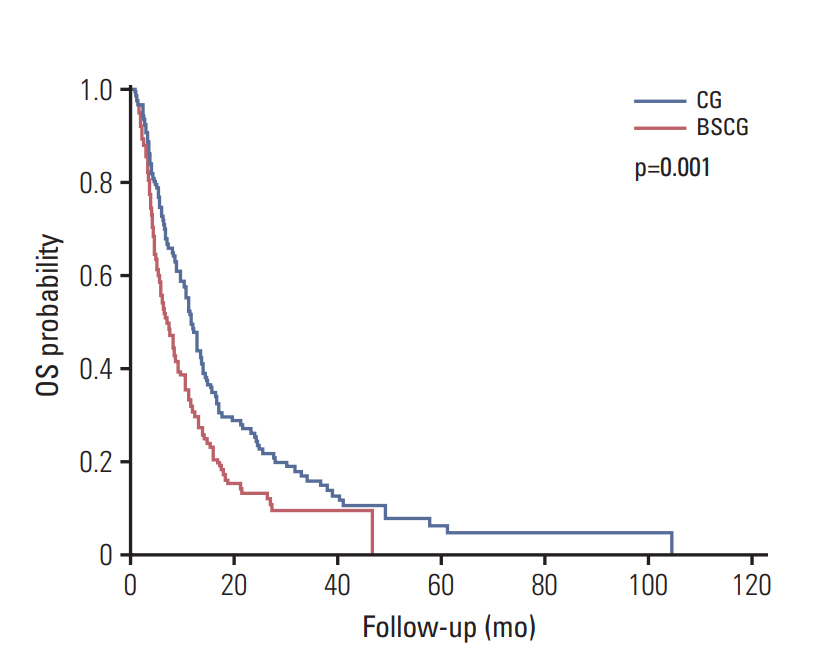
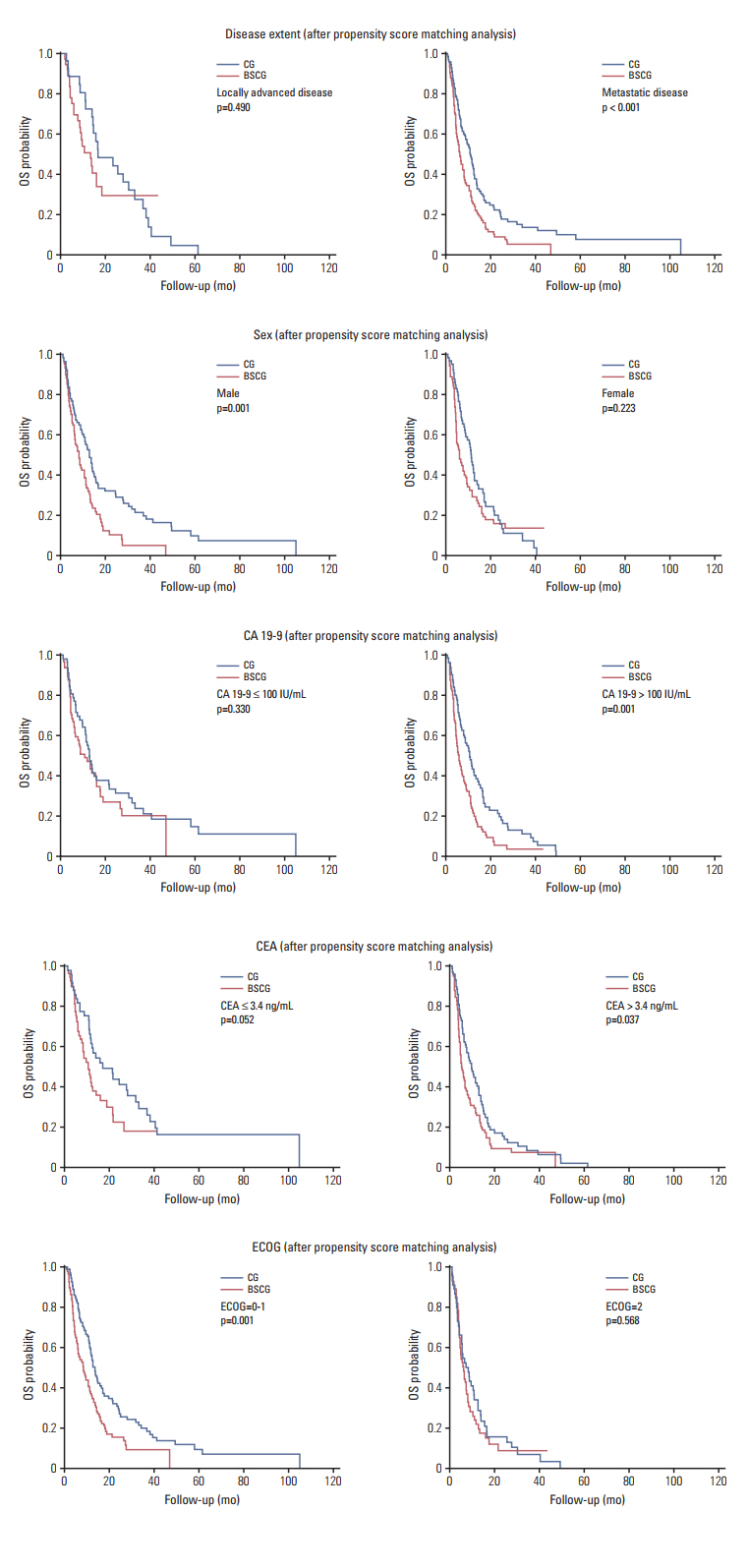
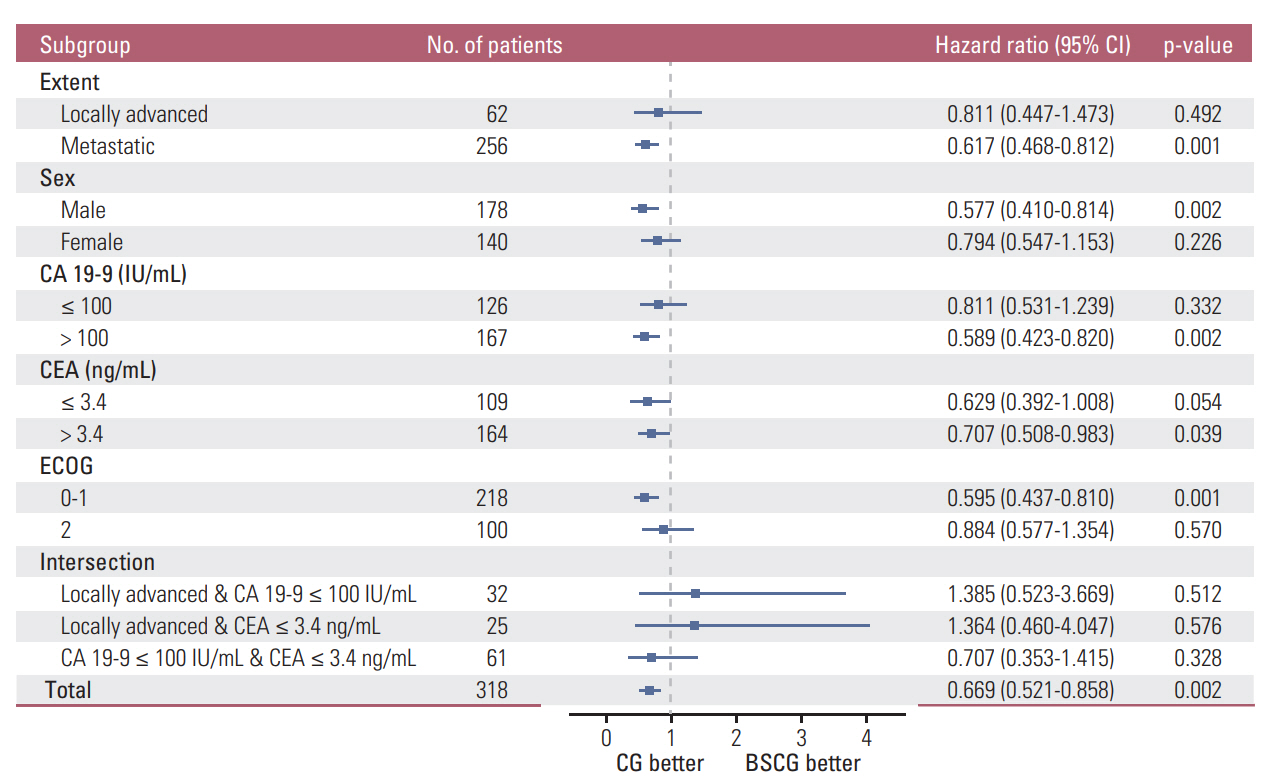
In post-PSM analysis, the respective median survival for the chemotherapy and BSC groups was dependent on the following prognostic factors: total population, 12.0 months vs. 7.5 months (p=0.001); locally advanced disease, 16.7 months vs. 13.4 months (p=0.490); cancer antigen 19-9 ≤ 100 IU/mL, 12.7 months vs. 10.6 months (p=0.330); and CEA ≤ 3.4 ng/mL, 17.1 months vs. 10.6 months (p=0.052).
CONCLUSION
Chemotherapy improved overall survival of patients with advanced BTC who had a good performance status. However, this survival benefit was not observed in BTC patients with locally advanced disease or with lower tumor marker. Individualized approach is needed for initiation of palliative chemotherapy in advanced BTC.
MeSH Terms
Figure
Reference
-
References
1. Huguier M, Barrier A, Valinas R, Flahault A, Adloff M, Pezet D, et al. Randomized trial of 5-fluorouracil, leucovorin and cisplatin in advanced pancreatic cancer. Hepatogastroenterology. 2001; 48:875–8.2. Mallinson CN, Rake MO, Cocking JB, Fox CA, Cwynarski MT, Diffey BL, et al. Chemotherapy in pancreatic cancer: results of a controlled, prospective, randomised, multicentre trial. Br Med J. 1980; 281:1589–91.
Article3. Palmer KR, Kerr M, Knowles G, Cull A, Carter DC, Leonard RC. Chemotherapy prolongs survival in inoperable pancreatic carcinoma. Br J Surg. 1994; 81:882–5.
Article4. Brieau B, Dahan L, De Rycke Y, Boussaha T, Vasseur P, Tougeron D, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: a large multicenter study by the Association des Gastro-Enterologues Oncologues. Cancer. 2015; 121:3290–7.5. Fornaro L, Vivaldi C, Cereda S, Leone F, Aprile G, Lonardi S, et al. Second-line chemotherapy in advanced biliary cancer progressed to first-line platinum-gemcitabine combination: a multicenter survey and pooled analysis with published data. J Exp Clin Cancer Res. 2015; 34:156.
Article6. Ji JH, Song HN, Kim RB, Oh SY, Lim HY, Park JO, et al. Natural history of metastatic biliary tract cancer (BTC) patients with good performance status (PS) who were treated with only best supportive care (BSC). Jpn J Clin Oncol. 2015; 45:256–60.
Article7. Valle JW, Wasan H, Johnson P, Jones E, Dixon L, Swindell R, et al. Gemcitabine alone or in combination with cisplatin in patients with advanced or metastatic cholangiocarcinomas or other biliary tract tumours: a multicentre randomised phase II study: The UK ABC-01 Study. Br J Cancer. 2009; 101:621–7.8. Park J, Kim MH, Kim KP, Park DH, Moon SH, Song TJ, et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: a large-scale observational study. Gut Liver. 2009; 3:298–305.
Article9. Sharma A, Dwary AD, Mohanti BK, Deo SV, Pal S, Sreenivas V, et al. Best supportive care compared with chemotherapy for unresectable gall bladder cancer: a randomized controlled study. J Clin Oncol. 2010; 28:4581–6.
Article10. Sjolander A. Propensity scores and M-structures. Stat Med. 2009; 28:1416–20.11. Ahmann DL, Green SJ, Bisel HF, Ingle JN, Hahn RG, Lee RA, et al. An evaluation of early or delayed adjuvant chemotherapy in premenopausal patients with advances breast cancer undergoing oophorectomy: a later analysis. Am J Clin Oncol. 1982; 5:355–8.12. Yu KD, Huang S, Zhang JX, Liu GY, Shao ZM. Association between delayed initiation of adjuvant CMF or anthracyclinebased chemotherapy and survival in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2013; 13:240.
Article13. Biagi JJ, Raphael MJ, Mackillop WJ, Kong W, King WD, Booth CM. Association between time to initiation of adjuvant chemotherapy and survival in colorectal cancer: a systematic review and meta-analysis. JAMA. 2011; 305:2335–42.14. Des Guetz G, Nicolas P, Perret GY, Morere JF, Uzzan B. Does delaying adjuvant chemotherapy after curative surgery for colorectal cancer impair survival? A meta-analysis. Eur J Cancer. 2010; 46:1049–55.
Article15. Gagliato Dde M, Gonzalez-Angulo AM, Lei X, Theriault RL, Giordano SH, Valero V, et al. Clinical impact of delaying initiation of adjuvant chemotherapy in patients with breast cancer. J Clin Oncol. 2014; 32:735–44.16. Ackland SP, Jones M, Tu D, Simes J, Yuen J, Sargeant AM, et al. A meta-analysis of two randomised trials of early chemotherapy in asymptomatic metastatic colorectal cancer. Br J Cancer. 2005; 93:1236–43.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Real-World Outcomes of Gemcitabine, Cisplatin, and Nab-Paclitaxel Chemotherapy Regimen for Advanced Biliary Tract Cancer: A Propensity Score-Matched Analysis
- Current status of chemotherapy for the treatment of advanced biliary tract cancer
- Efficacy and safety of cisplatin combined with paclitaxel concurrent radiotherapy in patients with locally advanced cervical squamous cell carcinoma
- Applications of propensity score matching: a case series of articles published in Annals of Coloproctology
- Effect of Biliary Drainage on the Prognosis of Patients with Hepatocellular Carcinoma and Bile Duct Invasion