J Pathol Transl Med.
2018 Jul;52(4):226-231. 10.4132/jptm.2017.11.12.
Hepatocellular Carcinoma Arising in a Huge Hepatocellular Adenoma with Bone Marrow Metaplasia
- Affiliations
-
- 1Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. Jihunkim@amc.seoul.kr
- 2Department of Physiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Asan Liver Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 4Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 5Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2417806
- DOI: http://doi.org/10.4132/jptm.2017.11.12
Abstract
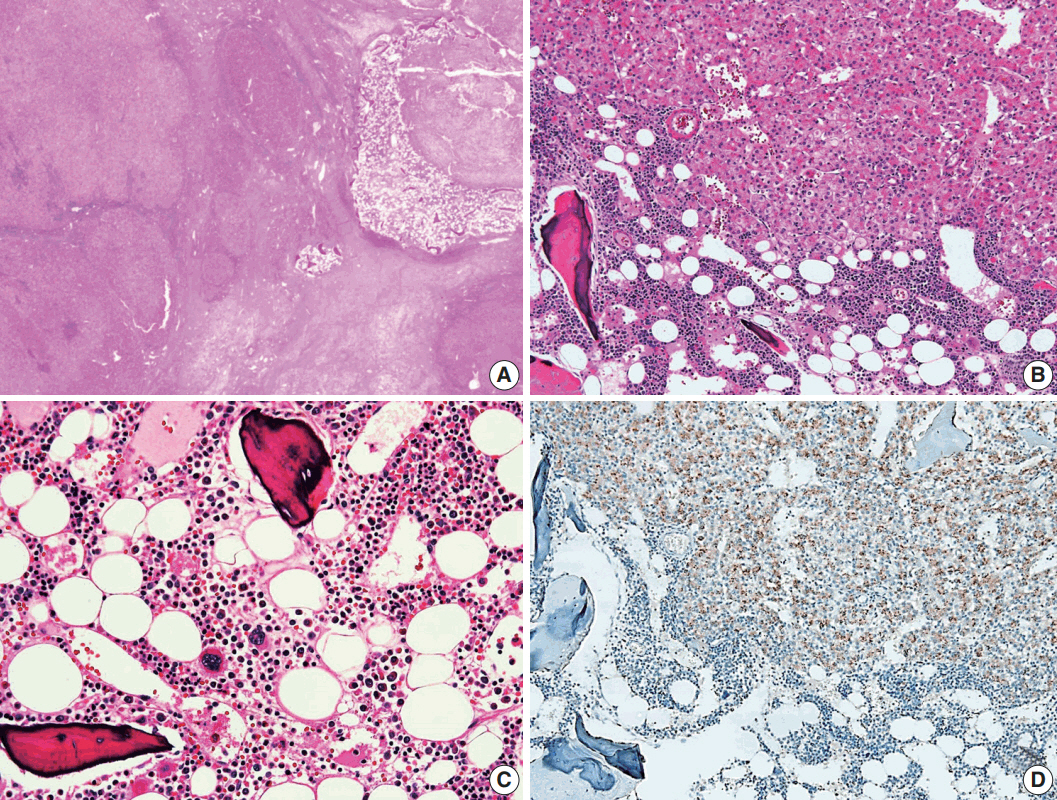
- Hepatocellular adenoma (HCA) is the most common type of benign liver tumor, and its major complication is malignant transformation to hepatocellular carcinoma (HCC). Here, we report a case of HCC arising in HCA with bone marrow metaplasia in a 24-year-old Korean woman who presented with abdominal discomfort. A huge liver mass was found on abdominal ultrasonography. She underwent surgical hepatic resection, and the resected specimen was entirely involved by a 20-cm-sized tumor. Histological review revealed a well differentiated HCC arising from inflammatory HCA with β-catenin nuclear positivity and bone marrow metaplasia that contained hematopoietic cells. This case was unique because malignant transformation, inflammatory type HCA, β-catenin nuclear staining, and bone marrow metaplasia were simultaneously observed. Additionally, it should be noted that a large HCA with β-catenin activation can undergo malignant transformation and should be surgically resected in a timely manner.
MeSH Terms
Figure
Reference
-
1. Chang CY, Hernandez-Prera JC, Roayaie S, Schwartz M, Thung SN. Changing epidemiology of hepatocellular adenoma in the United States: review of the literature. Int J Hepatol. 2013; 2013:604860.
Article2. Bioulac-Sage P, Sempoux C, Balabaud C. Hepatocellular adenoma: classification, variants and clinical relevance. Semin Diagn Pathol. 2017; 34:112–25.
Article3. Bioulac-Sage P, Balabaud C, Zucman-Rossi J. Subtype classification of hepatocellular adenoma. Dig Surg. 2010; 27:39–45.
Article4. Margolskee E, Bao F, de Gonzalez AK, et al. Hepatocellular adenoma classification: a comparative evaluation of immunohistochemistry and targeted mutational analysis. Diagn Pathol. 2016; 11:27.
Article5. Chu HH, Moon WS. β-catenin activated hepatocellular adenoma. Clin Mol Hepatol. 2013; 19:185–9.
Article6. Stoot JH, Coelen RJ, De Jong MC, Dejong CH. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford). 2010; 12:509–22.
Article7. Copin P, Ronot M, Vilgrain V. Hepatocellular carcinoma with osseous metaplasia and bone marrow elements. Clin Gastroenterol Hepatol. 2015; 13:e26–7.
Article8. Iguchi T, Yamagata M, Sonoda T, et al. Malignant transformation of hepatocellular adenoma with bone marrow metaplasia arising in glycogen storage disease type I: a case report. Mol Clin Oncol. 2016; 5:599–603.
Article9. Moriura S, Kuroda M, Kimura A, et al. Case report: hepatic adenoma with bone marrow metaplasia in a patient with glycogen storage disease type 1a. J Gastroenterol Hepatol. 1996; 11:556–9.
Article10. Ramacciato G, Nigri GR, Aurello P, et al. Giant hepatic adenoma with bone marrow metaplasia not associated with oral contraceptive intake. World J Surg Oncol. 2006; 4:58.
Article11. Vaithianathan R, Selvambigai G, Jayaganesh P. Spontaneous hepatocellular adenoma in paediatric age group: case report. J Clin Diagn Res. 2013; 7:2962–3.12. Velazquez I, Alter BP. Androgens and liver tumors: Fanconi’s anemia and non-Fanconi's conditions. Am J Hematol. 2004; 77:257–67.
Article13. Stueck AE, Qu Z, Huang MA, Campreciós G, Ferrell LD, Thung SN. Hepatocellular carcinoma arising in an HNF-1alpha-mutated adenoma in a 23-year-old woman with maturity-onset diabetes of the young: a case report. Semin Liver Dis. 2015; 35:444–9.14. Hirata E, Shimizu S, Umeda S, et al. Hepatocyte nuclear factor 1alpha-inactivated hepatocellular adenomatosis in a patient with maturity-onset diabetes of the young type 3: case report and literature review. Nihon Shokakibyo Gakkai Zasshi. 2015; 112:1696–704.15. Dokmak S, Paradis V, Vilgrain V, et al. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009; 137:1698–705.
Article16. Gorayski P, Thompson CH, Subhash HS, Thomas AC. Hepatocellular carcinoma associated with recreational anabolic steroid use. Br J Sports Med. 2008; 42:74–5.
Article17. Zucman-Rossi J, Jeannot E, Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006; 43:515–24.
Article18. Franco LM, Krishnamurthy V, Bali D, et al. Hepatocellular carcinoma in glycogen storage disease type Ia: a case series. J Inherit Metab Dis. 2005; 28:153–62.
Article19. Terkivatan T, de Wilt JH, de Man RA, et al. Indications and longterm outcome of treatment for benign hepatic tumors: a critical appraisal. Arch Surg. 2001; 136:1033–8.20. Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003; 102:3483–93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hepatocellular Carcinoma Arising in Hepatocellular Adenoma
- Hepatocellular Carcinoma Arising from Hepatocellular Adenoma in an Elderly Male Patient
- Atypical Nodule Arising in a Hepatocellular Adenoma
- Gelatinous transformation of the bone marrow in hepatocellular carcinoma
- A Case of a Patient Presenting with Upper Gastrointestinal Bleeding Due to Direct Stomach Invasion by Hepatocellular Carcinoma