J Vet Sci.
2018 Jul;19(4):550-556. 10.4142/jvs.2018.19.4.550.
Kilovoltage radiotherapy for companion animals: dosimetric comparison of 300 kV, 450 kV, and 6 MV X-ray beams
- Affiliations
-
- 1Department of Bio-Convergence Engineering, Korea University, Seoul 02841, Korea. radioyoon@korea.ac.kr
- 2Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul 03722, Korea. jinsung.k@gmail.com
- 3Korea Animal Cancer Center, Seoul 01684, Korea.
- 4Department of Radiation Oncology, Kyung Hee University Hospital at Gangdong, Seoul 05278, Korea.
- KMID: 2417571
- DOI: http://doi.org/10.4142/jvs.2018.19.4.550
Abstract
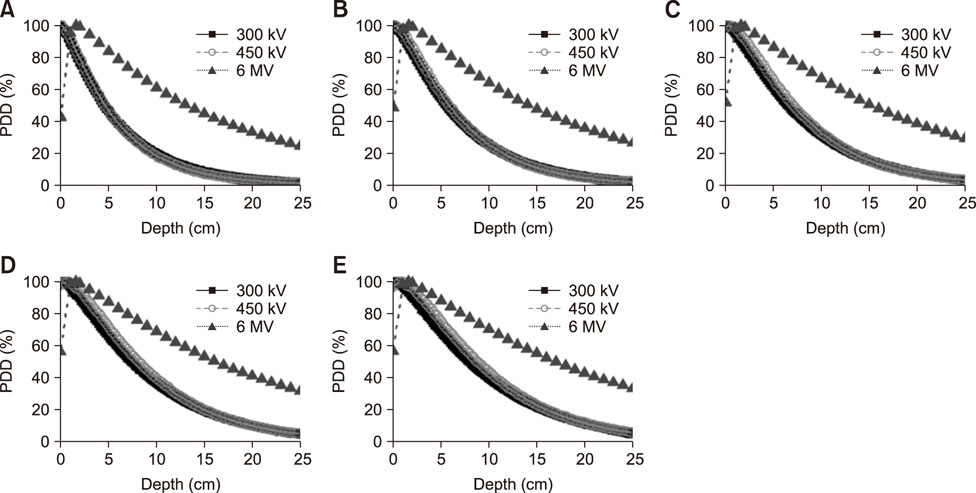
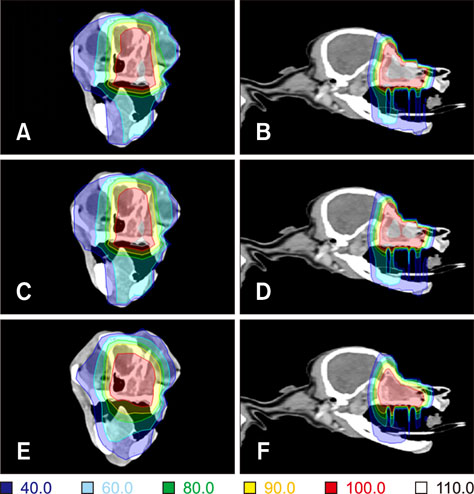
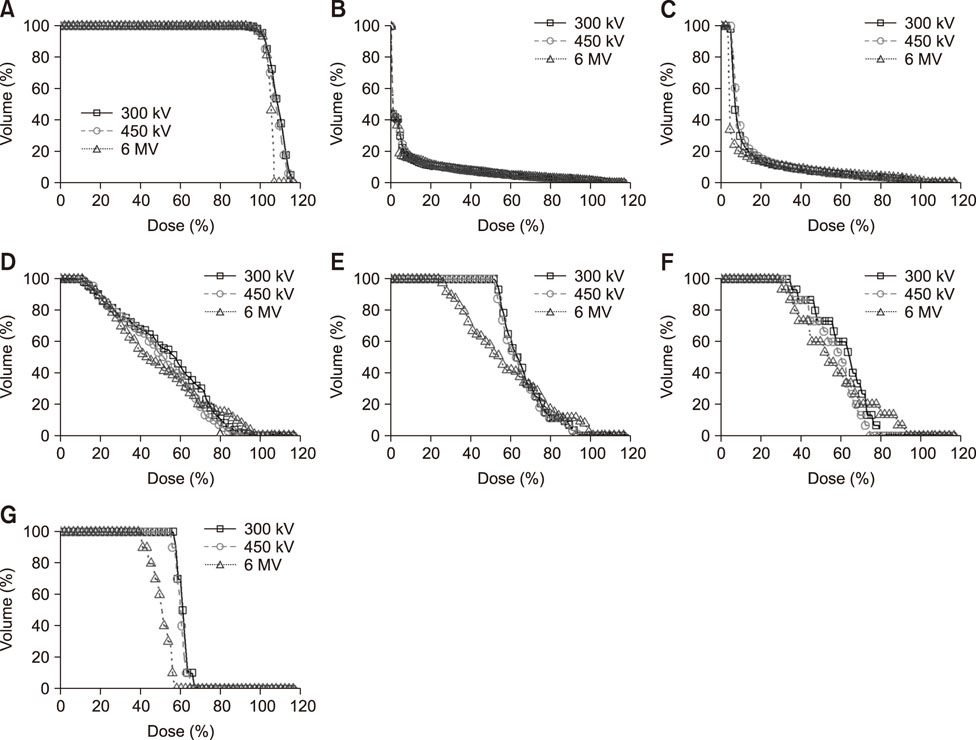
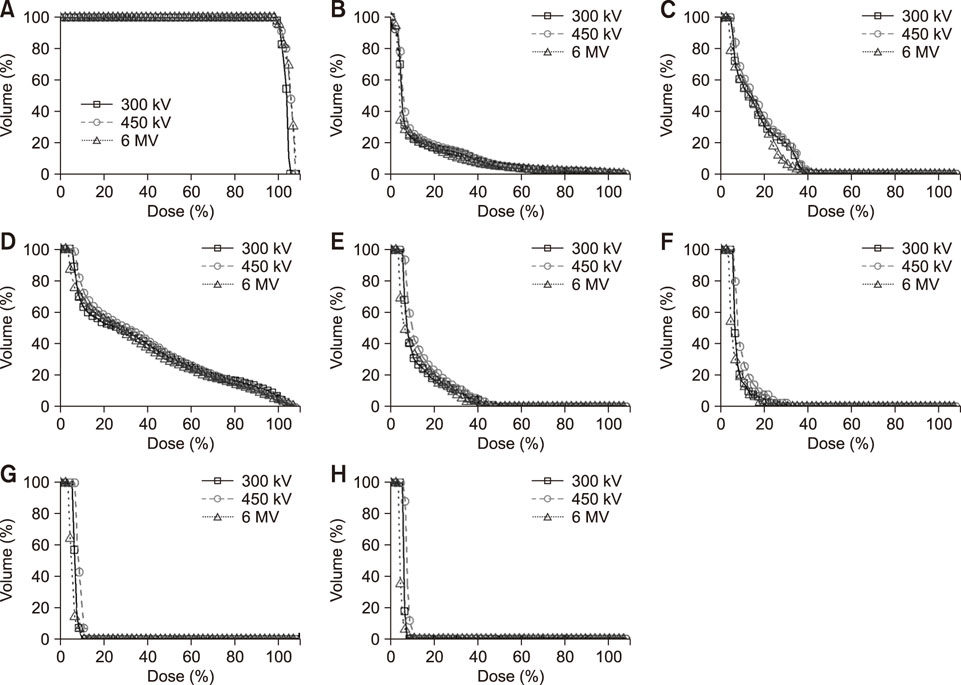
- Radiotherapy for the treatment of cancer in companion animals is currently administered by using megavoltage X-ray machines. Because these machines are expensive, most animal hospitals do not perform radiotherapy. This study evaluated the ability of relatively inexpensive kilovoltage X-ray machines to treat companion animals. A simulation study based on a commercial treatment-planning system was performed for tumors of the brain (non-infectious meningoencephalitis), nasal cavity (malignant nasal tumors), forefoot (malignant muscular tumors), and abdomen (malignant intestinal tumors). The results of kilovoltage (300 kV and 450 kV) and megavoltage (6 MV) X-ray beams were compared. Whereas the 300 kV and 6 MV X-ray beams provided optimal radiation dose homogeneity and conformity, respectively, for brain tumors, the 6 MV X-rays provided optimal homogeneity and radiation conformity for nasal cavity, forefoot, and abdominal tumors. Although megavoltage X-ray beams provided better radiation dose distribution in most treated animals, the differences between megavoltage and kilovoltage X-ray beams were relatively small. The similar therapeutic effects of the kilovoltage and 6 MV X-ray beams suggest that kilovoltage X-ray beams may be effective alternatives to megavoltage X-ray beams in treating cancers in companion animals.
Keyword
MeSH Terms
Figure
Reference
-
1. Austin-Seymour MM, Chen GT, Castro JR, Saunders WM, Pitluck S, Woodruff KH, Kessler M. Dose volume histogram analysis of liver radiation tolerance. Int J Radiat Oncol Biol Phys. 1986; 12:31–35.
Article2. Axlund TW, McGlasson ML, Smith AN. Surgery alone or in combination with radiation therapy for treatment of intracranial meningiomas in dogs: 31 cases (1989–2002). J Am Vet Med Assoc. 2002; 221:1597–1600.
Article3. Berlato D, Schrempp D, Van Den Steen N, Murphy S. Radiotherapy in the management of localized mucocutaneous oral lymphoma in dogs: 14 cases. Vet Comp Oncol. 2012; 10:16–23.
Article4. Blackwood L, Dobson J. Radiotherapy in small animal oncology. InPractice. 1998; 20:566–575.
Article5. Buvat I, Lazaro D. Monte Carlo simulations in emission tomography and GATE: an overview. Nucl Instrum Methods Phys Res A. 2006; 569:323–329.
Article6. Colvett K. The history of radiation oncology. South Med J. 2006; 99:1155–1156.
Article7. DeBoer DJ, Turrel JM, Moore PF. Mycosis fungoides in a dog: demonstration of T-cell specificity and response to radiotherapy. J Am Anim Hosp Assoc. 1990; 26:566–572.8. Dobson JM, Samuel S, Milstein H, Rogers K, Wood JL. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J Small Anim Pract. 2002; 43:240–246.
Article9. Dorn CR, Taylor DO, Schneider R, Hibbard HH, Klauber MR. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J Natl Cancer Inst. 1968; 40:307–331.10. Drzymala RE, Mohan R, Brewster L, Chu J, Goitein M, Harms W, Urie M. Dose-volume histograms. Int J Radiat Oncol Biol Phys. 1991; 21:71–78.
Article11. Gardner H, Fidel J, Haldorson G, Dernell W, Wheeler B. Canine oral fibrosarcomas: a retrospective analysis of 65 cases (1998–2010). Vet Comp Oncol. 2015; 13:40–47.
Article12. Henry CJ, Brewer WG Jr, Tyler JW, Brawner WR, Henderson RA, Hankes GH, Royer N. Survival in dogs with nasal adenocarcinoma: 64 cases (1981–1995). J Vet Intern Med. 1998; 12:436–439.
Article13. Hutson CA, Willauer CC, Walder EJ, Stone JL, Klein MK. Treatment of mandibular squamous cell carcinoma in cats by use of mandibulectomy and radiotherapy: seven cases (1987–1989). J Am Vet Med Assoc. 1992; 201:777–781.14. International Commission on Radiation Units and Measurements. Prescribing, Recording, and Reporting Photon Beam Therapy. Bethesda: International Commission on Radiation Units and Measurements;1993.15. International Commission on Radiation Units and Measurements. Prescribing, Recording, and Reporting Photon Beam Therapy. Bethesda: International Commission on Radiation Units and Measurements;1999.16. Jan S, Benoit D, Becheva E, Carlier T, Cassol F, Descourt P, Frisson T, Grevillot L, Guigues L, Maigne L, Morel C, Perrot Y, Rehfeld N, Sarrut D, Schaart DR, Stute S, Pietrzyk U, Visvikis D, Zahra N, Buvat I. GATE V6: a major enhancement of the GATE simulation platform enabling modelling of CT and radiotherapy. Phys Med Biol. 2011; 56:881–901.
Article17. Jung JY, Cho W, Kim MJ, Lee JW, Suh TS. Evaluation of beam modeling using collapsed cone convolution algorithm for dose calculation in radiation treatment planning system. Prog Med Phys. 2012; 23:188–198.18. Jung JY, Cho W, Lee JW, Suh TS. The experimental verification of collapsed cone convolution algorithm for dose calculation in radiation treatment planning system. IFMBE Proc. 2013; 39:1211–1214.
Article19. Khan FM, Gibbons JP. Khan's the Physics of Radiation Therapy. Philadelphia: Lippincott Williams & Wilkins;2014.20. MacEwen EG. Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer Metastasis Rev. 1990; 9:125–136.
Article21. Mesbahi A, Zakariaee SS. Effect of anode angle on photon beam spectra and depth dose characteristics for X-RAD320 orthovoltage unit. Rep Pract Oncol Radiother. 2013; 18:148–152.
Article22. Miceli A, Thierry R, Flisch A, Sennhauser U, Casali F, Simon M. Monte Carlo simulations of a high-resolution X-ray CT system for industrial applications. Nucl Instrum Methods Phys Res A. 2007; 583:313–323.
Article23. Nolan MW, Kogan L, Griffin LR, Custis JT, Harmon JF, Biller BJ, Larue SM. Intensity-modulated and image-guided radiation therapy for treatment of genitourinary carcinomas in dogs. J Vet Intern Med. 2012; 26:987–995.
Article24. Padikal TN, Deye JA. Electron contamination of a high-energy X-ray beam. Phys Med Biol. 1978; 23:1086–1092.
Article25. Pang LY, Argyle DJ. Veterinary oncology: biology, big data and precision medicine. Vet J. 2016; 213:38–45.
Article26. Poirier VJ, Bley CR, Roos M, Kaser-Hotz B. Efficacy of radiation therapy for the treatment of macroscopic canine oral soft tissue sarcoma. In Vivo. 2006; 20:415–419.27. Schiffman JD, Breen M. Comparative oncology: what dogs and other species can teach us about humans with cancer. Philos Trans R Soc Lond B Biol Sci. 2015; 370:20140231.
Article28. Shaw E, Kline R, Gillin M, Souhami L, Hirschfeld A, Dinapoli R, Martin L. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993; 27:1231–1239.
Article29. Strulab D, Santin G, Lazaro D, Breton V, Morel C. GATE (geant4 application for tomographic emission): a PET/SPECT general-purpose simulation platform. Nucl Phys B Proc Suppl. 2003; 125:75–79.
Article30. Thiam CO, Breton V, Donnarieix D, Habib B, Maigne L. Validation of a dose deposited by low-energy photons using GATE/GEANT4. Phys Med Biol. 2008; 53:3039–3055.
Article31. Wu Q, Mohan R, Morris M, Lauve A, Schmidt-Ullrich R. Simultaneous integrated boost intensity-modulated radiotherapy for locally advanced head-and-neck squamous cell carcinomas. I: dosimetric results. Int J Radiat Oncol Biol Phys. 2003; 56:573–585.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Determination of Quality Factors for Cylindrical Ionization Chambers in kV X-rays: Review of IAEA Dosimetry Protocol and Monte Carlo Calculations and Measurements for N23333 and N30001 Chambers
- Effect of the amount of battery charge on tube voltage in different hand-held dental x-ray systems
- Feasibility of Improving the Accuracy of Dose Calculation Using Hybrid Computed Tomography Images: A Phantom Study
- Inhibition of voltage-dependent K⺠current in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic drug propafenone
- Decreased voltage dependent K+ currents in cerebral arterial smooth muscle cells of one-kidney, one-clip Goldblatt hypertensive rat