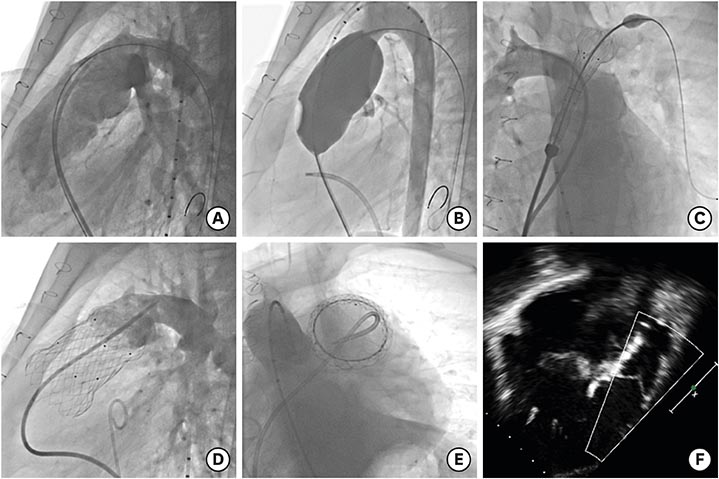
Korean Circ J.
2018 May;48(5):350-364. 10.4070/kcj.2018.0064.
Interventional Cardiology for Congenital Heart Disease
- Affiliations
-
- 1Our Lady's Children's Hospital, Crumlin, Dublin, Ireland. damien_kenny@icloud.com
- KMID: 2414905
- DOI: http://doi.org/10.4070/kcj.2018.0064
Abstract
- Congenital heart interventions are now replacing surgical palliation and correction in an evolving number of congenital heart defects. Right ventricular outflow tract and ductus arteriosus stenting have demonstrated favorable outcomes compared to surgical systemic to pulmonary artery shunting, and it is likely surgical pulmonary valve replacement will become an uncommon procedure within the next decade, mirroring current practices in the treatment of atrial septal defects. Challenges remain, including the lack of device design focused on smaller infants and the inevitable consequences of somatic growth. Increasing parental and physician expectancy has inevitably lead to higher risk interventions on smaller infants and appreciation of the consequences of these interventions on departmental outcome data needs to be considered. Registry data evaluating congenital heart interventions remain less robust than surgical registries, leading to a lack of insight into the longer-term consequences of our interventions. Increasing collaboration with surgical colleagues has not been met with necessary development of dedicated equipment for hybrid interventions aimed at minimizing the longer-term consequences of scar to the heart. Therefore, great challenges remain to ensure children and adults with congenital heart disease continue to benefit from an exponential growth in minimally invasive interventions and technology. This can only be achieved through a concerted collaborative approach from physicians, industry, academia and regulatory bodies supporting great innovators to continue the philosophy of thinking beyond the limits that has been the foundation of our specialty for the past 50 years.
MeSH Terms
Figure
Cited by 1 articles
-
The Significance of Self-Expandable Stents in Patients with Congenital Heart Disease in Current Era
Sang-Yun Lee
Korean Circ J. 2019;49(10):943-944. doi: 10.4070/kcj.2019.0201.
Reference
-
1. Velasco Forte MN, Byrne N, Valverde I, et al. Interventional correction of sinus venosus atrial septal defect and partial anomalous pulmonary venous drainage: procedural planning using 3D printed models. JACC Cardiovasc Imaging. 2018; 11:275–278.2. King TD, Thompson SL, Steiner C, Mills NL. Secundum atrial septal defect: nonoperative closure during cardiac catheterization. JAMA. 1976; 235:2506–2509.
Article3. Porstmann W, Wierny L, Warnke H. Der Vershuss des Ductus Arteriosus in persistens ohne Thorakotomie 81, Miiffeilung. Thoraxchirurgie. 1967; 15:109–203.4. Fu YC, Bass J, Amin Z, et al. Transcatheter closure of perimembranous ventricular septal defects using the new Amplatzer membranous VSD occluder: results of the U.S. phase I trial. J Am Coll Cardiol. 2006; 47:319–325.5. McElhinney DB, Quartermain MD, Kenny D, Alboliras E, Amin Z. Relative risk factors for cardiac erosion following transcatheter closure of atrial septal defects: a case-control study. Circulation. 2016; 133:1738–1746.6. van Velzen CL, Clur SA, Rijlaarsdam ME, et al. Prenatal detection of congenital heart disease--results of a national screening programme. BJOG. 2016; 123:400–407.
Article7. Maxwell D, Allan L, Tynan MJ. Balloon dilatation of the aortic valve in the fetus: a report of two cases. Br Heart J. 1991; 65:256–258.
Article8. Donofrio MT, Moon-Grady AJ, Hornberger LK, et al. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014; 129:2183–2242.9. Freud LR, McElhinney DB, Marshall AC, et al. Fetal aortic valvuloplasty for evolving hypoplastic left heart syndrome: postnatal outcomes of the first 100 patients. Circulation. 2014; 130:638–645.10. Marshall AC, Levine J, Morash D, et al. Results of in utero atrial septoplasty in fetuses with hypoplastic left heart syndrome. Prenat Diagn. 2008; 28:1023–1028.11. Emery SP, Kreutzer J, McCaffrey FM, Sherman FS, Simhan HN, Keller BB. The learning curve for a fetal cardiac intervention team. Minim Invasive Surg. 2010; 2010:674185.
Article12. Mohammad Nijres B, Kenny D, Kazmouz S, Hijazi ZM. Transcatheter closure of unroofed coronary sinus using covered stents in an adult with drainage of the coronary sinus to the right ventricle after supra-annular tricuspid valve replacement. Catheter Cardiovasc Interv. 2017; 90:1154–1157.
Article13. Papa M, Gaspardone A, Fragasso G, et al. Margonato. Feasibility and safety of transcatheter closure of atrial septal defects with deficient posterior rim. Catheter Cardiovasc Interv. 2013; 81:1180–1187.14. Gray RG, Menon SC, Johnson JT, et al. Acute and midterm results following perventricular device closure of muscular ventricular septal defects: a multicenter PICES investigation. Catheter Cardiovasc Interv. 2017; 90:281–289.
Article15. Koneti NR, Verma S, Bakhru S, et al. Transcatheter trans-septal antegrade closure of muscular ventricular septal defects in young children. Catheter Cardiovasc Interv. 2013; 82:E500–6.
Article16. Jameel AA, Arfi AM, Arif H, Amjad K, Omar GM. Retrograde approach for device closure of muscular ventricular septal defects in children and adolescents, using the Amplatzer muscular ventricular septal defect occluder. Pediatr Cardiol. 2006; 27:720–728.
Article17. Szkutnik M, Qureshi SA, Kusa J, Rosenthal E, Bialkowski J. Use of the Amplatzer muscular ventricular septal defect occluder for closure of perimembranous ventricular septal defects. Heart. 2007; 93:355–358.
Article18. Chungsomprasong P, Durongpisitkul K, Vijarnsorn C, Soongswang J, Lê TP. The results of transcatheter closure of VSD using Amplatzer® device and Nit Occlud® Lê coil. Catheter Cardiovasc Interv. 2011; 78:1032–1040.
Article19. Lin CH, Huddleston C, Balzer DT. Transcatheter ventricular septal defect (VSD) creation for restrictive VSD in double-outlet right ventricle. Pediatr Cardiol. 2013; 34:743–747.
Article20. Xu Z, Owens G, Gordon D, Cain C, Ludomirsky A. Noninvasive creation of an atrial septal defect by histotripsy in a canine model. Circulation. 2010; 121:742–749.
Article21. Backes CH, Cua C, Kreutzer J, et al. Low weight as an independent risk factor for adverse events during cardiac catheterization of infants. Catheter Cardiovasc Interv. 2013; 82:786–794.
Article22. Zahn EM, Peck D, Phillips A, et al. Transcatheter closure of patent ductus arteriosus in extremely premature newborns: early results and midterm follow-up. JACC Cardiovasc Interv. 2016; 9:2429–2437.23. Sathanandam S, Justino H, Waller BR 3rd, Radtke W, Qureshi AM. Initial clinical experience with the Medtronic Micro Vascular Plug™ in transcatheter occlusion of PDAs in extremely premature infants. Catheter Cardiovasc Interv. 2017; 89:1051–1058.
Article24. Akintuerk H, Michel-Behnke I, Valeske K, et al. Stenting of the arterial duct and banding of the pulmonary arteries: basis for combined Norwood stage I and II repair in hypoplastic left heart. Circulation. 2002; 105:1099–1103.25. Schranz D, Bauer A, Reich B, et al. Fifteen-year single center experience with the “Giessen Hybrid” approach for hypoplastic left heart and variants: current strategies and outcomes. Pediatr Cardiol. 2015; 36:365–373.
Article26. Karamlou T, Overman D, Hill KD, et al. Stage 1 hybrid palliation for hypoplastic left heart syndrome--assessment of contemporary patterns of use: an analysis of the Society of Thoracic Surgeons Congenital Heart Surgery Database. J Thorac Cardiovasc Surg. 2015; 149:195–201. 202.e1
Article27. Glatz AC, Petit CJ, Goldstein BH, et al. Comparison between patent ductus arteriosus stent and modified blalock-taussig shunt as palliation for infants with ductal-dependent pulmonary blood flow: insights from the congenital catheterization research collaborative. Circulation. 2018; 137:589–601.28. Bentham JR, Zava NK, Harrison WJ, et al. Duct stenting versus modified blalock taussig shunt in neonates with duct-dependent pulmonary blood flow: associations with clinical outcomes in a multicenter national study. Circulation. 2018; 137:581–588.29. Quandt D, Ramchandani B, Penford G, et al. Right ventricular outflow tract stent versus BT shunt palliation in tetralogy of Fallot. Heart. 2017; 103:1985–1991.
Article30. Quandt D, Ramchandani B, Stickley J, et al. Stenting of the right ventricular outflow tract promotes better pulmonary arterial growth compared with modified blalock-taussig shunt palliation in tetralogy of Fallot-type lesions. JACC Cardiovasc Interv. 2017; 10:1774–1784.
Article31. Barron DJ, Ramchandani B, Murala J, et al. Surgery following primary right ventricular outflow tract stenting for Fallot's tetralogy and variants: rehabilitation of small pulmonary arteries. Eur J Cardiothorac Surg. 2013; 44:656–662.
Article32. Neya K, Lee R, Guerrero JL, Lang P, Vlahakes GJ. Experimental ablation of outflow tract muscle with a thermal balloon catheter. Circulation. 1995; 91:2445–2453.
Article33. Ungerleider RM, Johnston TA, O'Laughlin MP, Jaggers JJ, Gaskin PR. Intraoperative stents to rehabilitate severely stenotic pulmonary vessels. Ann Thorac Surg. 2001; 71:476–481.
Article34. Patel S, Saini AP, Nair A, Weber HS. Transcarotid balloon valvuloplasty in neonates and small infants with critical aortic valve stenosis utilizing continuous transesophageal echocardiographic guidance: a 22 year single center experience from the cath lab to the bedside. Catheter Cardiovasc Interv. 2015; 86:821–827.
Article35. Sosnowski C, Matella T, Fogg L, et al. Hybrid pulmonary artery plication followed by transcatheter pulmonary valve replacement: comparison with surgical PVR. Catheter Cardiovasc Interv. 2016; 88:804–810.
Article36. Holzer RJ, Sisk M, Chisolm JL, et al. Completion angiography after cardiac surgery for congenital heart disease: complementing the intraoperative imaging modalities. Pediatr Cardiol. 2009; 30:1075–1082.
Article37. Takaya Y, Akagi T, Nakagawa K, Ito H. Integrated 3D echo-X-ray navigation guided transcatheter closure of complex multiple atrial septal defects. JACC Cardiovasc Interv. 2016; 9:e111–2.38. Aldoss O, Fonseca BM, Truong UT, et al. Diagnostic utility of three-dimensional rotational angiography in congenital cardiac catheterization. Pediatr Cardiol. 2016; 37:1211–1221.
Article39. Truong UT, Fagan TE, Deterding R, Ing RJ, Fonseca BM. Use of rotational angiography in assessing relationship of the airway to vasculature during cardiac catheterization. Catheter Cardiovasc Interv. 2015; 86:1068–1077.
Article40. Bruckheimer E, Rotschild C, Dagan T, et al. Computer-generated real-time digital holography: first time use in clinical medical imaging. Eur Heart J Cardiovasc Imaging. 2016; 17:845–849.
Article41. Cheatham JP, Hellenbrand WE, Zahn EM, et al. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015; 131:1960–1970.
Article42. Morray BH, McElhinney DB, Cheatham JP, et al. Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. Circ Cardiovasc Interv. 2013; 6:535–542.43. Torres A, Sanders SP, Vincent JA, et al. Iatrogenic aortopulmonary communications after transcatheter interventions on the right ventricular outflow tract or pulmonary artery: pathophysiologic, diagnostic, and management considerations. Catheter Cardiovasc Interv. 2015; 86:438–452.
Article44. Van Dijck I, Budts W, Cools B, et al. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart. 2015; 101:788–793.
Article45. Boudjemline Y. A new one-step procedure for pulmonary valve implantation of the melody valve: simultaneous prestenting and valve implantation. Catheter Cardiovasc Interv. 2018; 91:64–70.
Article46. Shahanavaz S, Rockefeller T, Nicolas R, Balzer D. Fracturing a dysfunctional Edwards Perimount bioprosthetic valve to facilitate percutaneous valve-in-valve placement of SAPIEN 3 valve with modified delivery system. Catheter Cardiovasc Interv. 2018; 91:81–85.
Article47. Morray BH, McElhinney DB, Boudjemline Y, et al. Multicenter experience evaluating transcatheter pulmonary valve replacement in bovine jugular vein (Contegra) right ventricle to pulmonary artery conduits. Circ Cardiovasc Interv. 2017; 10:e004914.
Article48. Levi DS, Sinha S, Salem MM, Aboulhosn JA. Transcatheter native pulmonary valve and tricuspid valve replacement with the sapien XT: initial experience and development of a new delivery platform. Catheter Cardiovasc Interv. 2016; 88:434–443.
Article49. Cao QL, Kenny D, Zhou D, et al. Early clinical experience with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2014; 84:1131–1137.
Article50. Bergersen L, Benson LN, Gillespie MJ, et al. Harmony feasibility trial: acute and short-term outcomes with a self-expanding transcatheter pulmonary valve. JACC Cardiovasc Interv. 2017; 10:1763–1773.51. Kim GB, Kwon BS, Lim HG. First in human experience of a new self-expandable percutaneous pulmonary valve implantation using knitted nitinol-wire and tri-leaflet porcine pericardial valve in the native right ventricular outflow tract. Catheter Cardiovasc Interv. 2017; 89:906–909.
Article52. Phillips AB, Nevin P, Shah A, Olshove V, Garg R, Zahn EM. Development of a novel hybrid strategy for transcatheter pulmonary valve placement in patients following transannular patch repair of tetralogy of Fallot. Catheter Cardiovasc Interv. 2016; 87:403–410.
Article53. Hasan BS, McElhinney DB, Brown DW, et al. Short-term performance of the transcatheter melody valve in high-pressure hemodynamic environments in the pulmonary and systemic circulations. Circ Cardiovasc Interv. 2011; 4:615–620.
Article54. Quiñonez LG, Breitbart R, Tworetsky W, Lock JE, Marshall AC, Emani SM. Stented bovine jugular vein graft (melody valve) for surgical mitral valve replacement in infants and children. J Thorac Cardiovasc Surg. 2014; 148:1443–1449.
Article55. Maglione J, Bergersen L, Lock JE, McElhinney DB. Ultra-high-pressure balloon angioplasty for treatment of resistant stenoses within or adjacent to previously implanted pulmonary arterial stents. Circ Cardiovasc Interv. 2009; 2:52–58.
Article56. Ewert P, Riesenkampff E, Neuss M, Kretschmar O, Nagdyman N, Lange PE. Novel growth stent for the permanent treatment of vessel stenosis in growing children: an experimental study. Catheter Cardiovasc Interv. 2004; 62:506–510.
Article57. Shibbani K, Kenny D, McElhinney D, Hijazi ZM, Moran T. Identifying gaps in technology for congenital interventions: analysis of a needs survey from congenital interventional cardiologists. Pediatr Cardiol. 2016; 37:925–931.
Article58. McCrossan BA, McMahon CJ, Walsh KP. First reported use of drug-eluting bioabsorbable vascular scaffold in congenital heart disease. Catheter Cardiovasc Interv. 2016; 87:324–328.
Article59. Mullen MJ, Hildick-Smith D, De Giovanni JV, et al. BioSTAR Evaluation STudy (BEST): a prospective, multicenter, phase I clinical trial to evaluate the feasibility, efficacy, and safety of the BioSTAR bioabsorbable septal repair implant for the closure of atrial-level shunts. Circulation. 2006; 114:1962–1967.60. Happel CM, Laser KT, Sigler M, Kececioglu D, Sandica E, Haas NA. Single center experience: Implantation failures, early, and late complications after implantation of a partially biodegradable ASD/PFO-device (BioStar®). Catheter Cardiovasc Interv. 2015; 85:990–997.
Article61. Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008; 118:2395–2451.62. Feltes TF, Bacha E, Beekman RH 3rd, et al. Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011; 123:2607–2652.63. Porras D, Brown DW, Rathod R, et al. Acute outcomes after introduction of a standardized clinical assessment and management plan (SCAMP) for balloon aortic valvuloplasty in congenital aortic stenosis. Congenit Heart Dis. 2014; 9:316–325.
Article64. Ryan A, Duignan S, Kenny D, McMahon CJ. Decision making in paediatric cardiology. Are we prone to heuristics, biases and traps? Pediatr Cardiol. 2018; 39:160–167.
Article65. Boe BA, Zampi JD, Kennedy KF, et al. Acute success of balloon aortic valvuloplasty in the current era: an NCDR® study. JACC Cardiovasc Interv. 2017; 10:1717–1726.66. Bergersen L, Gauvreau K, Foerster SR, et al. Catheterization for congenital heart disease adjustment for risk method (CHARM). JACC Cardiovasc Interv. 2011; 4:1037–1046.
Article67. Nykanen DG, Forbes TJ, Du W, et al. CRISP: Catheterization RISk score for Pediatrics: a report from the Congenital Cardiac Interventional Study Consortium (CCISC). Catheter Cardiovasc Interv. 2016; 87:302–309.
Article68. Cevallos PC, Rose MJ, Armsby LB, et al. Implementation of methodology for quality improvement in pediatric cardiac catheterization: a multi-center initiative by the congenital cardiac catheterization project on outcomes-quality improvement (C3PO-QI). Pediatr Cardiol. 2016; 37:1436–1445.
Article69. Cohen S, Liu A, Gurvitz M, et al. Exposure to low-dose ionizing radiation from cardiac procedures and malignancy risk in adults with congenital heart disease. [Epub ahead of print]. Circulation. 2017.70. Esch JJ, Shah PB, Cockrill BA, et al. Transcatheter Potts shunt creation in patients with severe pulmonary arterial hypertension: initial clinical experience. J Heart Lung Transplant. 2013; 32:381–387.
Article71. Alsoufi B, Alfadley F, Al-Omrani A, et al. Hybrid management strategy for percutaneous Fontan completion without surgery: early results. Ann Thorac Surg. 2011; 91:566–572.
Article72. Ratnayaka K, Rogers T, Schenke WH, et al. Magnetic resonance imaging-guided transcatheter cavopulmonary shunt. JACC Cardiovasc Interv. 2016; 9:959–970.
Article73. Rogers T, Lederman RJ. Interventional CMR: clinical applications and future directions. Curr Cardiol Rep. 2015; 17:31.
Article74. Sizarov A, Boudjemline Y. Novel materials and devices in the transcatheter management of congenital heart diseases--the future comes slowly (part 1). Arch Cardiovasc Dis. 2016; 109:278–285.75. Sizarov A, Boudjemline Y. Novel materials and devices in the transcatheter creation of vascular anastomosis--the future comes slowly (part 2). Arch Cardiovasc Dis. 2016; 109:286–295.76. Sizarov A, Boudjemline Y. Novel materials and devices in the transcatheter management of congenital heart diseases-the future comes slowly (part 3). Arch Cardiovasc Dis. 2016; 109:348–358.
Article77. Haggerty CM, de Zélicourt DA, Restrepo M, et al. Comparing pre- and post-operative Fontan hemodynamic simulations: implications for the reliability of surgical planning. Ann Biomed Eng. 2012; 40:2639–2651.
Article78. Brili S, Tousoulis D, Antoniades C, et al. Evidence of vascular dysfunction in young patients with successfully repaired coarctation of aorta. Atherosclerosis. 2005; 182:97–103.
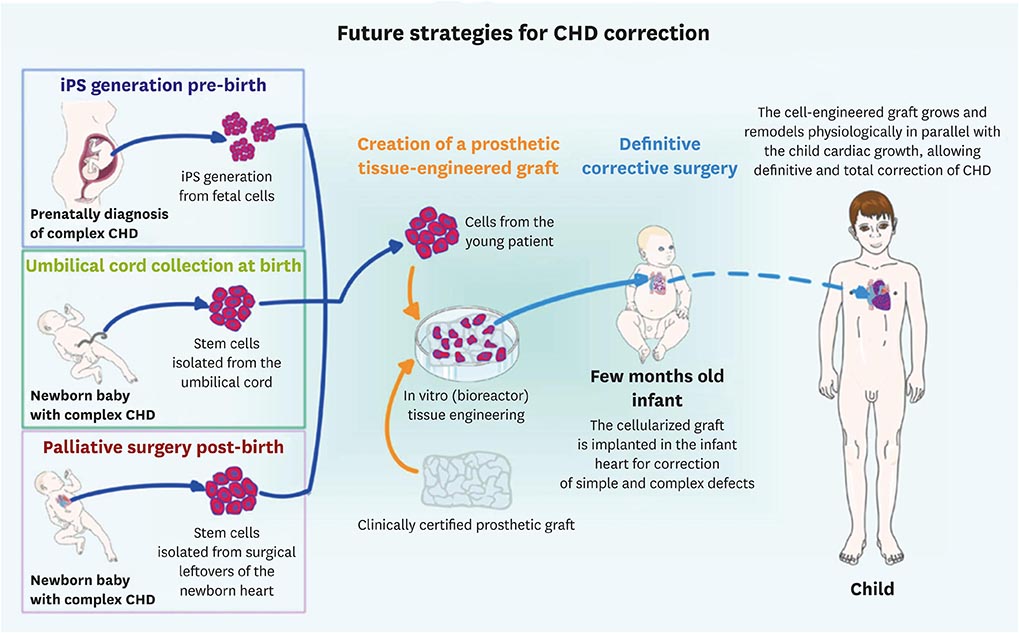
Article79. Frydrychowicz A, Markl M, Harloff A, et al. Flow-sensitive in-vivo 4D MR imaging at 3T for the analysis of aortic hemodynamics and derived vessel wall parameters. RoFo Fortschr Geb Rontgenstr Nuklearmed. 2007; 179:463–472.80. Avolio E, Caputo M, Madeddu P. Stem cell therapy and tissue engineering for correction of congenital heart disease. Front Cell Dev Biol. 2015; 3:39.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent advances in pediatric interventional cardiology
- Interventional Treatment of Congenital Heart Disease
- Differential Diagnosis of Congenital Heart Diseases
- Recent Trend of Increasing Proportion of Interventional Catheterization in Congenital Heart Disease
- Diastolic Dysfunction in Congenital Heart Disease