J Gastric Cancer.
2018 Mar;18(1):69-81. 10.5230/jgc.2018.18.e9.
Survival Benefit of Perioperative Chemotherapy in Patients with Locally Advanced Gastric Cancer: a Propensity Score Matched Analysis
- Affiliations
-
- 1Center for Gastric Cancer, Research Institute and Hospital, National Cancer Center, Goyang, Korea. docryu@ncc.re.kr, srpark@amc.seoul.kr
- 2Biometric Research Branch, Research Institute for National Cancer Control and Evaluation, National Cancer Center, Goyang, Korea.
- 3Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2414548
- DOI: http://doi.org/10.5230/jgc.2018.18.e9
Abstract
- PURPOSE
It has been reported that the survival of patients with locally advanced gastric cancer (LAGC) is better in East Asia countries than in developed western countries; however, the prognosis of LAGC remains poor. This study aimed to evaluate the effects of perioperative chemotherapy on the long-term survival of East Asia patients with LAGC.
MATERIALS AND METHODS
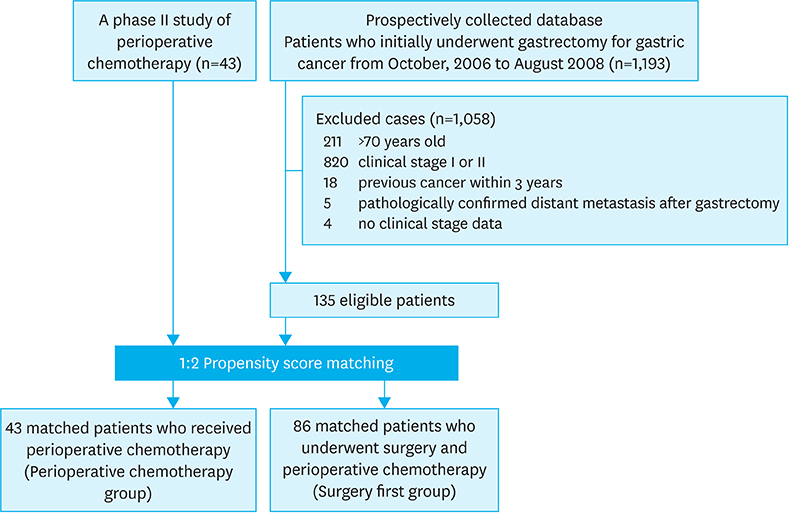
From October 2006 through August 2008, 43 patients with LAGC received perioperative S-1 combined with weekly docetaxel in a phase II study (neoadjuvant group). These patients were matched using propensity scores to patients who underwent surgery without neoadjuvant chemotherapy during the same period (surgery group). The surgical outcomes and long-term survivals were compared between the 2 groups.
RESULTS
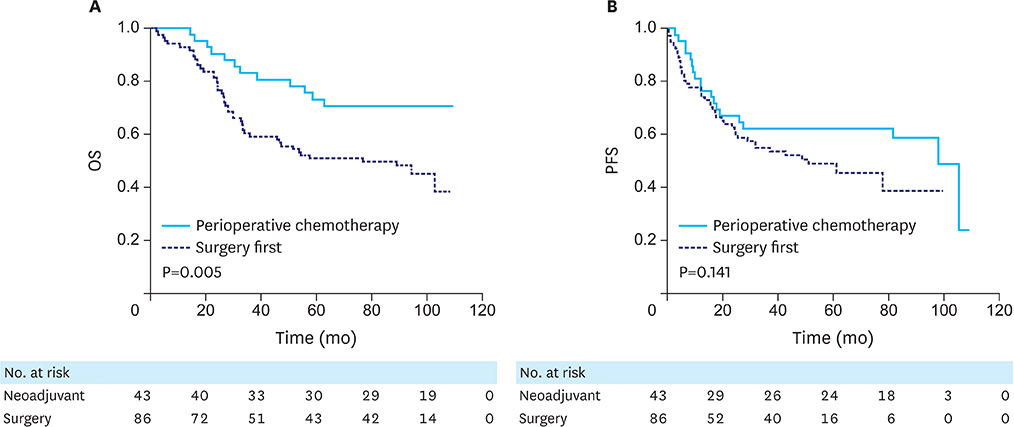
After matching, 43 and 86 patients were included in the neoadjuvant and surgery groups, respectively, and there was no significant difference in their baseline characteristics. Although the operating time was longer in the neoadjuvant group, there was no significant difference in postoperative complications between the 2 groups. The neoadjuvant group had a significantly higher 5-year overall survival (OS) rate (73.3% vs. 51.1%, P=0.005) and a trend towards higher 5-year progression-free survival (PFS) (62.8% vs. 49.9%, P=0.145). In the multivariate analysis, perioperative chemotherapy was an independent factor for OS, with a hazard ratio of 0.4 (P=0.005) and a marginal effect on the PFS (P=0.054).
CONCLUSIONS
Perioperative chemotherapy was associated with better long-term survival without increasing postoperative complications in the setting of D2 surgery for patients with LAGC, suggesting that perioperative chemotherapy can be a therapeutic option in East Asia countries.
MeSH Terms
Figure
Reference
-
1. Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, et al. Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram. Ann Surg. 2010; 251:640–646.
Article2. Jeong O, Park YK. Clinicopathological features and surgical treatment of gastric cancer in South Korea: the results of 2009 nationwide survey on surgically treated gastric cancer patients. J Gastric Cancer. 2011; 11:69–77.
Article3. Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, et al. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013; 16:1–27.
Article4. Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006; 355:11–20.
Article5. Ychou M, Boige V, Pignon JP, Conroy T, Bouche O, Lebreton G, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol. 2011; 29:1715–1721.
Article6. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820.
Article7. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–321.
Article8. Yoon HM, Ryu KW, Nam BH, Cho SJ, Park SR, Lee JY, et al. Is the new seventh AJCC/UICC staging system appropriate for patients with gastric cancer? J Am Coll Surg. 2012; 214:88–96.
Article9. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:1389–1396.
Article10. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011; 29:4387–4393.
Article11. Chun J, Park S, Kim H, Kim Y, Ryu K, Lee J, et al. Randomized phase II trial of neoadjuvant vs. adjuvant docetaxel plus cisplatin in patients with locally advanced gastric carcinoma: an interim analysis. J Clin Oncol. 2006; 24:suppl. abstr 4030.
Article12. Kim YW, Kim MJ, Ryu KW, Lim HS, Lee JH, Kong SY, et al. A phase II study of perioperative S-1 combined with weekly docetaxel in patients with locally advanced gastric carcinoma: clinical outcomes and clinicopathological and pharmacogenetic predictors for survival. Gastric Cancer. 2016; 19:586–596.
Article13. Fujitani K, Sasako M, Iwasaki Y, Yoshimura K, Sano T, Nashimoto A, et al. A phase II study of preoperative chemotherapy (CX) with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancer: JCOG 0210. J Clin Oncol. 2007; 25:suppl. abstr 4609.
Article14. Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009; 96:1015–1022.
Article15. Yoshikawa T, Nakamura K, Tsuburaya A, Sano T, Mizusawa J, Katai H, et al. A phase II study of preoperative chemotherapy with S-1 (S) and cisplatin (P) followed by D3 gastrectomy for gastric cancer (GC) with extensive lymph node metastasis (ELM): survival results of JCOG 0405. J Clin Oncol. 2011; 29:suppl. abstr 70.16. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 2nd English edition. Gastric Cancer. 1998; 1:10–24.17. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000; 92:205–216.18. Kim SK, Kang KW, Lee JS, Kim HK, Chang HJ, Choi JY, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging. 2006; 33:148–155.
Article19. Nakajima T. Gastric cancer treatment guidelines in Japan. Gastric Cancer. 2002; 5:1–5.
Article20. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373–383.
Article21. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992; 45:613–619.
Article22. Aaltonen LA, Hamilton SR. World Health Organization. Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press;2000.23. Sobin LH. TNM Classification of Malignant Tumours. 6th ed. New York (NY): John Wiley;2002.24. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004; 240:205–213.25. Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011; 10:150–161.
Article26. Park I, Ryu MH, Choi YH, Kang HJ, Yook JH, Park YS, et al. A phase II study of neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) chemotherapy followed by surgery and adjuvant S-1 chemotherapy in potentially resectable gastric or gastroesophageal junction adenocarcinoma. Cancer Chemother Pharmacol. 2013; 72:815–823.
Article27. Kosaka T, Akiyama H, Makino H, Takagawa R, Kimura J, Ono H, et al. Preoperative S-1 and docetaxel combination chemotherapy in patients with locally advanced gastric cancer. Cancer Chemother Pharmacol. 2014; 73:281–285.
Article28. Hirakawa M, Sato Y, Ohnuma H, Takayama T, Sagawa T, Nobuoka T, et al. A phase II study of neoadjuvant combination chemotherapy with docetaxel, cisplatin, and S-1 for locally advanced resectable gastric cancer: nucleotide excision repair (NER) as potential chemoresistance marker. Cancer Chemother Pharmacol. 2013; 71:789–797.
Article29. Takiguchi N, Nunomura M, Koda K, Oda K, Suzuki H, Miyazaki M. Neoadjuvant chemotherapy with CDDP and 5-fluorouracil for gastric cancer with serosal invasion. Oncol Rep. 2003; 10:433–438.
Article30. Kang YK, Choi DW, Im YH, Kim CM, Lee JI, Moon NM, et al. A phase III randomized comparison of neoadjuvant chemotherapy followed by surgery versus surgery for locally advanced stomach cancer. J Clin Oncol. 1996; 15:suppl. abstr 503.31. Li ZY, Shan F, Zhang LH, Bu ZD, Wu AW, Wu XJ, et al. Complications after radical gastrectomy following FOLFOX7 neoadjuvant chemotherapy for gastric cancer. World J Surg Oncol. 2011; 9:110.
Article32. Ahn HS, Jeong SH, Son YG, Lee HJ, Im SA, Bang YJ, et al. Effect of neoadjuvant chemotherapy on postoperative morbidity and mortality in patients with locally advanced gastric cancer. Br J Surg. 2014; 101:1560–1565.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant Chemotherapy in Asian Patients With Locally Advanced Gastric Cancer
- Preoperative Chemotherapy in Advanced Stomach Cancer (Cons)
- Preoperative Chemotherapy in Advanced Stomach Cancer (Pros)
- Postoperative Radiotherapy Improves Survival in Gastric Signet-Ring Cell Carcinoma: a SEER Database Analysis
- Effect of Early Adjuvant Chemotherapy on Survival of Advanced Gastric Cancer Patients: a Propensity Score-matched Analysis