J Gastric Cancer.
2018 Mar;18(1):48-57. 10.5230/jgc.2018.18.e4.
The Prognostic Significance of Compliance with Postoperative Adjuvant Chemotherapy in Patients with Stage III Gastric Cancer: an Observational Study
- Affiliations
-
- 1Department of Surgery, Hanyang University Seoul Hospital, Hanyang University School of Medicine, Seoul, Korea. sjkwon@hanyang.ac.kr
- 2Department of Surgery, Hanyang University Guri Hospital, Hanyang University School of Medicine, Guri, Korea.
- KMID: 2414546
- DOI: http://doi.org/10.5230/jgc.2018.18.e4
Abstract
- PURPOSE
Postoperative adjuvant chemotherapy is usually prescribed to improve the survival of patients with advanced gastric cancer who undergo curative surgery. This study was designed to determine the impact that the degree of compliance with chemotherapy has on the prognosis of patients with gastric cancer.
MATERIALS AND METHODS
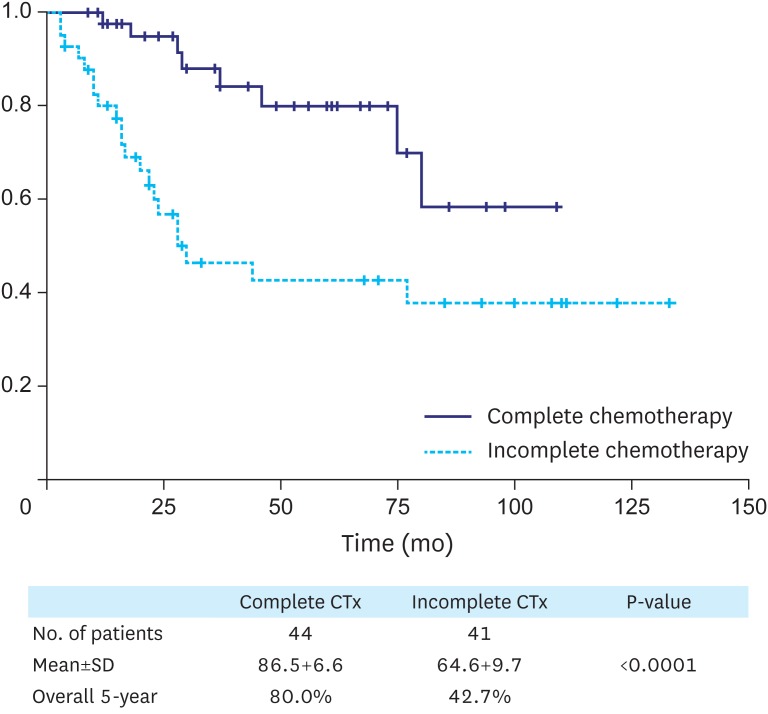
Among 252 patients with stage III gastric cancer who underwent curative surgery between July 2004 and December 2014, 85 patients were postoperatively treated with S-1, the oral fluoropyrimidine derivative, 23 received no chemotherapy, and 144 received other regimens. Overall survival was compared between the complete compliance group (who received 8 cycles of S-1 chemotherapy, n=44) and the incomplete compliance group (who received less than 8 cycles of S-1 chemotherapy, n=41). Factors that influenced patient compliance with chemotherapy were also analyzed.
RESULTS
The overall 5-year survival rate was significantly different between the complete chemotherapy and incomplete chemotherapy groups (80.0% vs. 42.7%, P<0.001). Based on univariate and multivariate survival analyses of patients who received S-1 chemotherapy, the independent prognostic factors were tumor, node, and metastasis (TNM) stage (IIIa vs. IIIb vs. IIIc) and compliance with chemotherapy. TNM stage and age are significant factors that influence compliance with chemotherapy.
CONCLUSIONS
TNM stage and compliance with chemotherapy are independent prognostic factors in patients with stage III gastric cancer who received postoperative chemotherapy. TNM stage and age are significant factors that influence patient compliance with chemotherapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Rugge M, Fassan M, Graham D. Epidemiology of gastric cancer. In : Strong VE, editor. Gastric Cancer: Principles and Practice. Cham: Springer;2015. p. 23–34.2. Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol. 2014; 20:1635–1649. PMID: 24587643.
Article3. Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2010; CD004064. PMID: 20238327.
Article4. Kilic L, Ordu C, Yildiz I, Sen F, Keskin S, Ciftci R, et al. Current adjuvant treatment modalities for gastric cancer: from history to the future. World J Gastrointest Oncol. 2016; 8:439–449. PMID: 27190583.
Article5. Fuentes E, Ahmad R, Hong TS, Clark JW, Kwak EL, Rattner DW, et al. Adjuvant therapy completion rates in patients with gastric cancer undergoing perioperative chemotherapy versus a surgery-first approach. J Gastrointest Surg. 2016; 20:172–179. PMID: 26394879.
Article6. Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KW, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy CLASSIC: a phase 3 open-label, randomised controlled trial. Lancet. 2012; 379:315–321. PMID: 22226517.
Article7. Diaz-Nieto R, Orti-Rodríguez R, Winslet M. Post-surgical chemotherapy versus surgery alone for resectable gastric cancer. Cochrane Database Syst Rev. 2013; CD008415. PMID: 23999923.
Article8. Lee JH, Kim JG, Jung HK, Kim JH, Jeong WK, Jeon TJ, et al. Clinical practice guidelines for gastric cancer in Korea: an evidence-based approach. J Gastric Cancer. 2014; 14:87–104. PMID: 25061536.
Article9. Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy CLASSIC: 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014; 15:1389–1396. PMID: 25439693.
Article10. Miceli R, Tomasello G, Bregni G, Di Bartolomeo M, Pietrantonio F. Adjuvant chemotherapy for gastric cancer: current evidence and future challenges. World J Gastroenterol. 2014; 20:4516–4525. PMID: 24782604.
Article11. Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017; 20:1–19.12. Foo M, Leong T. Adjuvant therapy for gastric cancer: current and future directions. World J Gastroenterol. 2014; 20:13718–13727. PMID: 25320509.
Article13. Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, et al. Survival analysis of adjuvant chemotherapy with S-1 plus cisplatin for stage III gastric cancer. Gastric Cancer. 2014; 17:383–386. PMID: 23719867.
Article14. Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor REGATTA: a phase 3, randomised controlled trial. Lancet Oncol. 2016; 17:309–318. PMID: 26822397.
Article15. Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011; 29:4387–4393. PMID: 22010012.
Article16. Zhao SL, Fang JY. The role of postoperative adjuvant chemotherapy following curative resection for gastric cancer: a meta-analysis. Cancer Invest. 2008; 26:317–325. PMID: 18317973.
Article17. Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011; 60:1449–1472. PMID: 21705456.
Article18. Saif MW, Makrilia N, Zalonis A, Merikas M, Syrigos K. Gastric cancer in the elderly: an overview. Eur J Surg Oncol. 2010; 36:709–717. PMID: 20542657.
Article19. Imamura H, Kishimoto T, Takiuchi H, Kimura Y, Morimoto T, Imano M, et al. Phase II study of S-1 monotherapy in patients over 75 years of age with advanced gastric cancer (OGSG0404). J Chemother. 2014; 26:57–61. PMID: 24090674.
Article20. Eun H, Hur H, Byun CS, Son SY, Han SU, Cho YK. Effects of continuing adjuvant S-1 for 1 year on the prognosis of gastric cancer patients: results from a prospective single center study. J Gastric Cancer. 2015; 15:113–120. PMID: 26161284.
Article21. Aoyama T, Yoshikawa T, Hayashi T, Kuwabara H, Mikayama Y, Ogata T, et al. Risk factors for 6-month continuation of S-1 adjuvant chemotherapy for gastric cancer. Gastric Cancer. 2013; 16:133–139. PMID: 22527186.
Article22. Aoyama T, Sato T, Maezawa Y, Kano K, Hayashi T, Yamada T, et al. Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int J Clin Oncol. 2017; 22:476–483. PMID: 28176023.
Article23. Yamashita K, Kurokawa Y, Yamamoto K, Hirota M, Kawabata R, Mikami J, et al. Risk factors for poor compliance with adjuvant S-1 chemotherapy for gastric cancer: a multicenter retrospective study. Ann Surg Oncol. 2017; 24:2639–2645. PMID: 28608116.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Update of Adjuvant Chemotherapy for Resected Gastric Cancer
- Immunochemosurgery for Gastric Carcinoma
- The Comparison of Survival Rates of Postoperative Adjuvant Chemotherapies in The Stage III Gastric Cancer Patients
- Preoperative Chemotherapy in Advanced Stomach Cancer (Pros)
- Survival Rates after Operation for Gastric Cancer: Fifteen-year Experience at a Korea Cancer Center Hospital