J Breast Cancer.
2016 Sep;19(3):275-282. 10.4048/jbc.2016.19.3.275.
Radiation Pneumonitis in Association with Internal Mammary Node Irradiation in Breast Cancer Patients: An Ancillary Result from the KROG 08-06 Study
- Affiliations
-
- 1Department of Radiation Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. COSUH317@yuhs.ac
- 2Department of Radiation Oncology, Proton Therapy Center, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- 3Department of Radiation Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 4Department of Radiation Oncology, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea.
- 5Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Department of Radiation Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 7Department of Radiation Oncology, Dongsan Medical Center, Keimyung University School of Medicine, Daegu, Korea.
- 8Department of Radiation Oncology, Gachon University Gil Medical Center, Incheon, Korea.
- 9Department of Radiation Oncology, Pusan National University Hospital, Pusan National University School of Medicine, Busan, Korea.
- 10Department of Radiation Oncology, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- 11Department of Radiation Oncology, CHA Bundang Hospital, CHA University College of Medicine, Seongnam, Korea.
- KMID: 2413952
- DOI: http://doi.org/10.4048/jbc.2016.19.3.275
Abstract
- PURPOSE
The aim of this study is to present the incidence of radiation pneumonitis (RP) reported within 6 months after treatment for breast cancer with or without internal mammary node irradiation (IMNI).
METHODS
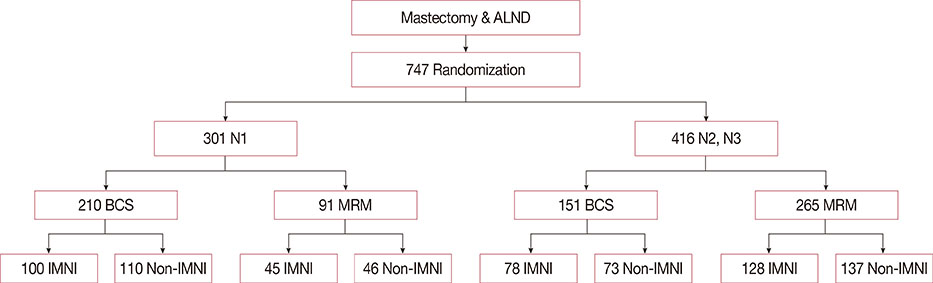
In the Korean Radiation Oncology Group (KROG) 08-06 phase III randomized trial, patients who were node-positive after surgery were randomly assigned to receive radiotherapy either with or without IMNI. A total of 747 patients were enrolled, and three-dimensional treatment planning with computed tomography simulation was performed for all patients. Of the 747 patients, 722 underwent chest X-rays before and within 6 months after radiotherapy. These 722 patients underwent evaluation, and RP was diagnosed on the basis of chest radiography findings and clinical symptoms. The relationship between the incidence of RP and clinical/dosimetric parameters was analyzed.
RESULTS
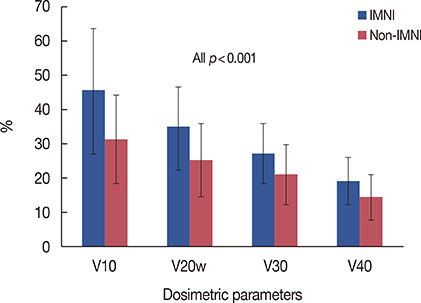
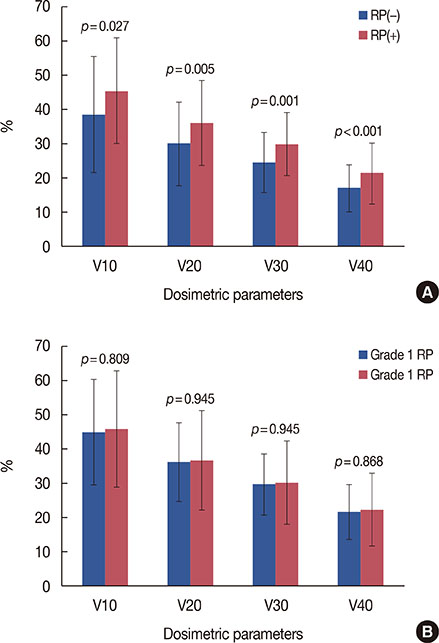
RP developed in 35 patients (4.8%), including grade 1 RP in 26 patients (3.6%), grade 2 RP in nine patients (1.2%); there was no incidence of grade 3 or higher RP. Grade 2 RP cases were observed in only the IMNI group. The risk of developing RP was influenced by IMNI treatment; pneumonitis occurred in 6.5% of patients (n=23/356) who underwent IMNI and in 3.3% of patients (n=12/366) who did not (p=0.047). The differences in lung dosimetric parameters (mean lung dose, V10-40) were statistically significant between the two groups.
CONCLUSION
IMNI treatment resulted in increased radiation exposure to the lung and a higher rate of RP, but the incidence and severity of RP was minimal and acceptable. This minor impact on morbidity should be balanced with the impact on survival outcome in future analyses.
MeSH Terms
Figure
Reference
-
1. Keum KC, Shim SJ, Lee IJ, Park W, Lee SW, Shin HS, et al. The 1998, 1999 patterns of care study for breast irradiation after mastectomy in Korea. J Korean Soc Ther Radiol Oncol. 2007; 25:7–15.2. Chargari C, Castadot P, Macdermed D, Vandekerkhove C, Bourgois N, Van Houtte P, et al. Internal mammary lymph node irradiation contributes to heart dose in breast cancer. Med Dosim. 2010; 35:163–168.
Article3. Taghian A, Jagsi R, Makris A, Goldberg S, Ceilley E, Grignon L, et al. Results of a survey regarding irradiation of internal mammary chain in patients with breast cancer: practice is culture driven rather than evidence based. Int J Radiat Oncol Biol Phys. 2004; 60:706–714.
Article4. Freedman GM, Fowble BL, Nicolaou N, Sigurdson ER, Torosian MH, Boraas MC, et al. Should internal mammary lymph nodes in breast cancer be a target for the radiation oncologist? Int J Radiat Oncol Biol Phys. 2000; 46:805–814.
Article5. Matzinger O, Heimsoth I, Poortmans P, Collette L, Struikmans H, Van Den Bogaert W, et al. Toxicity at three years with and without irradiation of the internal mammary and medial supraclavicular lymph node chain in stage I to III breast cancer (EORTC trial 22922/10925). Acta Oncol. 2010; 49:24–34.
Article6. Whelan TJ, Olivotto I, Ackerman I, Chapman JW, Chua B, Nabid A, et al. NCIC-CTG MA. 20: an intergroup trial of regional nodal irradiation in early breast cancer. J Clin Oncol. 2011; 29:15 Suppl. LBA1003.7. Overgaard M, Hansen PS, Overgaard J, Rose C, Andersson M, Bach F, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy: Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997; 337:949–955.
Article8. Chander SS. Postoperative radiotherapy in high-risk postmenopausal breast cancer: authors' reply. Lancet. 1999; 354:865–866.9. Chang JS, Park W, Kim YB, Lee IJ, Keum KC, Lee CG, et al. Long-term survival outcomes following internal mammary node irradiation in stage II-III breast cancer: results of a large retrospective study with 12-year follow-up. Int J Radiat Oncol Biol Phys. 2013; 86:867–872.
Article10. Kubo A, Osaki K, Kawanaka T, Furutani S, Ikushima H, Nishitani H. Risk factors for radiation pneumonitis caused by whole breast irradiation following breast-conserving surgery. J Med Invest. 2009; 56:99–110.
Article11. Lingos TI, Recht A, Vicini F, Abner A, Silver B, Harris JR. Radiation pneumonitis in breast cancer patients treated with conservative surgery and radiation therapy. Int J Radiat Oncol Biol Phys. 1991; 21:355–360.
Article12. Arthur DW, Arnfield MR, Warwicke LA, Morris MM, Zwicker RD. Internal mammary node coverage: an investigation of presently accepted techniques. Int J Radiat Oncol Biol Phys. 2000; 48:139–146.
Article13. Jeong K, Shim SJ, You SH, Kim YB, Keum KC, Kim JD, et al. A study of the radiotherapy techniques for the breast including internal mammary lymph nodes. J Korean Soc Ther Radiol Oncol. 2009; 27:35–41.
Article14. Kim HJ, Jang WI, Kim TJ, Kim JH, Kim SW, Moon SH, et al. Radiation-induced pulmonary toxicity and related risk factors in breast cancer. J Breast Cancer. 2009; 12:67–72.
Article15. Chung Y, Yoon HI, Kim YB, Ahn SK, Keum KC, Suh CO. Radiation pneumonitis in breast cancer patients who received radiotherapy using the partially wide tangent technique after breast conserving surgery. J Breast Cancer. 2012; 15:337–343.
Article16. Krengli M, Sacco M, Loi G, Masini L, Ferrante D, Gambaro G, et al. Pulmonary changes after radiotherapy for conservative treatment of breast cancer: a prospective study. Int J Radiat Oncol Biol Phys. 2008; 70:1460–1467.
Article17. Muren LP, Maurstad G, Hafslund R, Anker G, Dahl O. Cardiac and pulmonary doses and complication probabilities in standard and conformal tangential irradiation in conservative management of breast cancer. Radiother Oncol. 2002; 62:173–183.
Article18. Das IJ, Cheng EC, Freedman G, Fowble B. Lung and heart dose volume analyses with CT simulator in radiation treatment of breast cancer. Int J Radiat Oncol Biol Phys. 1998; 42:11–19.
Article19. Lind PA, Wennberg B, Gagliardi G, Rosfors S, Blom-Goldman U, Lideståhl A, et al. ROC curves and evaluation of radiation-induced pulmonary toxicity in breast cancer. Int J Radiat Oncol Biol Phys. 2006; 64:765–770.
Article20. Prosnitz RG, Chen YH, Marks LB. Cardiac toxicity following thoracic radiation. Semin Oncol. 2005; 32:2 Suppl 3. S71–S80.
Article21. Prosnitz RG, Hubbs JL, Evans ES, Zhou SM, Yu X, Blazing MA, et al. Prospective assessment of radiotherapy-associated cardiac toxicity in breast cancer patients: analysis of data 3 to 6 years after treatment. Cancer. 2007; 110:1840–1850.
Article22. Harris EE. Cardiac mortality and morbidity after breast cancer treatment. Cancer Control. 2008; 15:120–129.
Article23. Nilsson G, Holmberg L, Garmo H, Terent A, Blomqvist C. Radiation to supraclavicular and internal mammary lymph nodes in breast cancer increases the risk of stroke. Br J Cancer. 2009; 100:811–816.
Article24. Bartlett FR, Colgan RM, Carr K, Donovan EM, McNair HA, Locke I, et al. The UK HeartSpare Study: randomised evaluation of voluntary deep-inspiratory breath-hold in women undergoing breast radiotherapy. Radiother Oncol. 2013; 108:242–247.
Article25. Mast ME, van Kempen-Harteveld L, Heijenbrok MW, Kalidien Y, Rozema H, Jansen WP, et al. Left-sided breast cancer radiotherapy with and without breath-hold: does IMRT reduce the cardiac dose even further? Radiother Oncol. 2013; 108:248–253.
Article26. Lee HY, Chang JS, Lee IJ, Park K, Kim YB, Suh CO, et al. The deep inspiration breath hold technique using Abches reduces cardiac dose in patients undergoing left-sided breast irradiation. Radiat Oncol J. 2013; 31:239–246.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Internal Mammary Lymph Node Irradiation after Breast Conservation Surgery: Radiation Pneumonitis versus Dose?Volume Histogram Parameters
- Radiation Pneumonitis in Breast Cancer Patients Who Received Radiotherapy Using the Partially Wide Tangent Technique after Breast Conserving Surgery
- A Study of the Radiotherapy Techniques for the Breast Including Internal Mammary Lymph Nodes
- Bilateral Diffuse Radiation Pneumonitis Caused by Unilateral Thoracic Irradiation: A Case Report
- The Studies on the Development of Radiation Pneumonitis and Its Related Factors