Blood Res.
2017 Jun;52(2):130-134. 10.5045/br.2017.52.2.130.
Comparison of the characteristics of two hemoglobin variants, Hb D-Iran and Hb E, eluting in the Hb A2 window
- Affiliations
-
- 1Department of Hematology, Sir Ganga Ram Hospital, New Delhi, India. draasthagupta83@gmail.com
- KMID: 2413331
- DOI: http://doi.org/10.5045/br.2017.52.2.130
Abstract
- BACKGROUND
Cation exchange-high performance liquid chromatography (CE-HPLC) is most commonly used to evaluate hemoglobin (Hb) variants, which elute in the Hb A2 window. This study aimed to assess prevalence of an uncommon Hb variant, Hb D-Iran, and compare its red cell parameters and peak characteristics with those of Hb E that commonly elutes in the Hb A2 window.
METHODS
Generally, we assess abnormal Hb using CE-HPLC as the primary technique along with alkaline and acid electrophoresis. All cases with Hb A2 window >9%, as assessed by CE-HPLCs during 2009-2013, were selected.
RESULTS
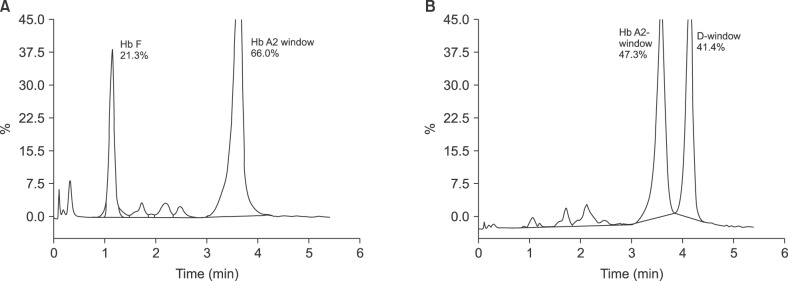
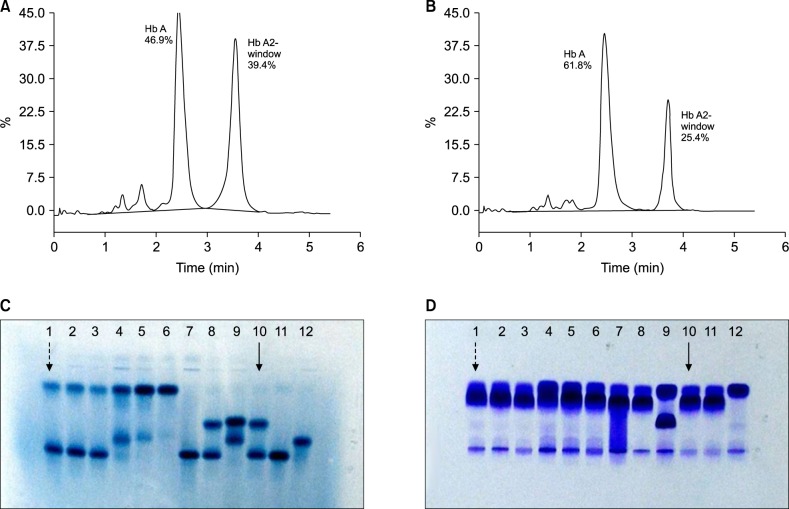
Twenty-nine cases with Hb D-Iran variant were identified"”25 heterozygous, 2 homozygous, 1 compound heterozygous Hb D-Iran/β-thalassemia, and 1 Hb D-Iran/Hb D-Punjab. Overall prevalence of Hb D-Iran was 0.23%. Compared to patients with Hb E, those with Hb D-Iran had significantly higher Hb (12.1 vs. 11.3 g/dL, P=0.03), MCV (82.4 vs. 76.4 fL, P=0.0044), MCH (27.9 vs. 25.45 pg, P =0.0006), and MCHC (33.9 vs. 33.3 g/dL, P=0.0005). Amount of abnormal Hb (40.7 vs. 26.4%, P=0.0001) was significantly higher while retention time (3.56 vs. 3.70 min, P=0.0001) was significantly lower in Hb D-Iran than in Hb E.
CONCLUSION
Hb D-Iran peak can be easily missed if area and retention time of the Hb A2 window are not carefully analyzed. To distinguish between variants, careful analysis of peak area and retention time is sufficient in most cases and may be further confirmed by the second technique"”alkaline electrophoresis.
Keyword
Figure
Reference
-
1. Rahbar S. Haemoglobin D Iran: β222 glutamic acid leads to glutamine (B4). Br J Haematol. 1973; 24:31–35. PMID: 4715135.2. Wajcman H, Moradkhani K. Abnormal haemoglobins: detection & characterization. Indian J Med Res. 2011; 134:538–546. PMID: 22089618.3. Colah RB, Surve R, Sawant P, et al. HPLC studies in hemoglobinopathies. Indian J Pediatr. 2007; 74:657–662. PMID: 17699975.
Article4. Rao S, Kar R, Gupta SK, Chopra A, Saxena R. Spectrum of haemoglobinopathies diagnosed by cation exchange-HPLC & modulating effects of nutritional deficiency anaemias from north India. Indian J Med Res. 2010; 132:513–519. PMID: 21150000.5. Olivieri NF, Pakbaz Z, Vichinsky E. HbE/β-thalassemia: basis of marked clinical diversity. Hematol Oncol Clin North Am. 2010; 24:1055–1070. PMID: 21075280.
Article6. Sachdev R, Dam AR, Tyagi G. Detection of Hb variants and hemoglobinopathies in Indian population using HPLC: report of 2600 cases. Indian J Pathol Microbiol. 2010; 53:57–62. PMID: 20090224.
Article7. Ryan K, Bain BJ, Worthington D, et al. Significant haemoglobinopathies: guidelines for screening and diagnosis. Br J Haematol. 2010; 149:35–49. PMID: 20067565.
Article8. Joutovsky A, Hadzi-Nesic J, Nardi MA. HPLC retention time as a diagnostic tool for hemoglobin variants and hemoglobinopathies: a study of 60000 samples in a clinical diagnostic laboratory. Clin Chem. 2004; 50:1736–1747. PMID: 15388656.
Article9. Tyagi S, Pati HP, Choudhry VP, Saxena R. Clinico-haematological profile of HbE syndrome in adults and children. Hematology. 2004; 9:57–60. PMID: 14965869.
Article10. Mondal SK, Dasgupta S, Mondal S, Das N. Spectrum of thalassemias and hemoglobinopathies in West Bengal: A study of 90,210 cases by cation exchange high-performance liquid chromatography method over a period of 8 years. J Appl Hematol. 2014; 5:91–95.
Article11. Zakerinia M, Ayatollahi M, Rastegar M, et al. Hemoglobin D (Hb D Punjab/Los Angeles and Hb D Iran) and co-inheritance with alpha- and beta-thalassemia in southern Iran. Iran Red Crescent Med J. 2011; 13:493–498.12. Bhat VS, Mandal AK, Mathew B. Co-inheritance of HbD (Iran)/beta thalassemia IVS1-5 (G > C) trait in a punjabi lady with diabetes. Indian J Clin Biochem. 2012; 27:202–206. PMID: 23543793.13. Agrawal MG, Bhanushali AA, Dedhia P, et al. Compound heterozygosity of Hb D(Iran) (beta(22) Glu-->Gln) and beta(0)-thalassemia (619 bp-deletion) in India. Eur J Haematol. 2007; 79:248–250. PMID: 17655708.14. Gupta A, Saraf A, Dass J, et al. Compound heterozygous hemoglobin d-punjab/hemoglobin d-iran: a novel hemoglobinopathy. Indian J Hematol Blood Transfus. 2014; 30(Suppl 1):409–412. PMID: 25332633.
Article15. Serjeant B, Myerscough E, Serjeant GR, Higgs DR, Moo-Penn WF. Sickle cell-hemoglobin D Iran: benign sickle cell syndrome. Hemoglobin. 1982; 6:57–59. PMID: 7073867.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Accuracy of continuous and real-time total hemoglobin during bimaxillary orthognathic surgery
- Role of iron deficiency anemia in the propagation of beta thalssemia gene
- Comparative analysis of cellulose acetate hemoglobin electrophoresis and high performance liquid chromatography for quantitative determination of hemoglobin A2
- Hematologic Characteristics and Hemoglobin Fraction Analysis by High Performance Liquid Chromatogaphy in Patients with Hypochromic Microcytosis: Trials for Detection of beta-thalassemia
- Hydroxyurea Therapy in Hemoglobin Madrid Patients