J Vet Sci.
2016 Dec;17(4):435-444. 10.4142/jvs.2016.17.4.435.
Therapeutic effect of topical application of curcumin during treatment of radiation burns in a mini-pig model
- Affiliations
-
- 1Laboratory of Radiation Exposure & Therapeutics, Korea Institute of Radiological & Medical Sciences (KIRAMS), Seoul 01812, Korea. sslee@kcch.re.kr jskim@dirams.re.kr
- 2Research Center, Dongnam Institute of Radiological & Medical Sciences (DIRAMS), Busan 46033, Korea.
- 3Department of Dermatology, Korea Institute of Radiological & Medical Sciences (KIRAMS), Seoul 01812, Korea.
- 4College of Oriental Medicine, Dongshin Univiersity, Naju 58245, Korea.
- KMID: 2412598
- DOI: http://doi.org/10.4142/jvs.2016.17.4.435
Abstract
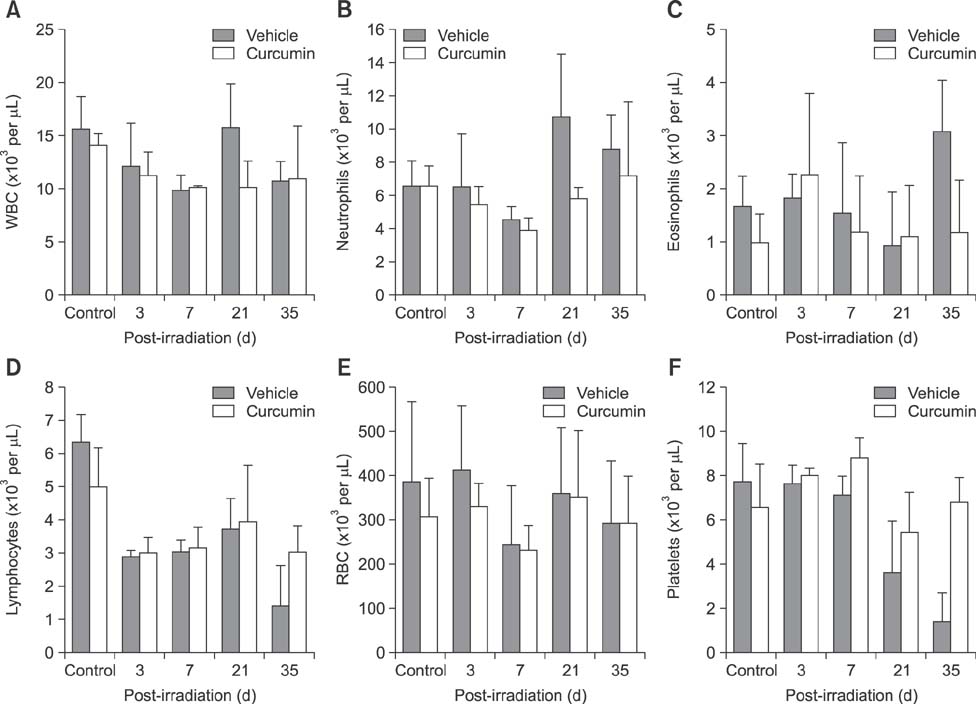
- Curcumin protects the skin against radiation-induced epidermal damage and prevents morphological changes induced by irradiation skin, thereby maintaining the epidermal thickness and cell density of basal layers. In this study, the effects of topical curcumin treatment on radiation burns were evaluated in a mini-pig model. Histological and clinical changes were observed five weeks after radiation exposure to the back (â¶â°Co gamma-radiation, 50 Gy). Curcumin was applied topically to irradiated skin (200 mg/cm²) twice a day for 35 days. Curcumin application decreased the epithelial desquamation after irradiation. Additionally, when compared to the vehicle-treated group, the curcumin-treated group showed reduced expression of cyclooxygenase-2 and nuclear factor-kappaB. Furthermore, irradiation prolonged healing of biopsy wounds in the exposed area, whereas curcumin treatment stimulated wound healing. These results suggest that curcumin can improve epithelial cell survival and recovery in the skin and therefore be used to treat radiation burns.
Keyword
MeSH Terms
-
Administration, Topical
Animals
Burns/*drug therapy
Curcumin/*pharmacology/*therapeutic use
Gamma Rays/*adverse effects
Gene Expression Regulation/drug effects
Radiation-Protective Agents/pharmacology/*therapeutic use
Skin/drug effects/*radiation effects
Swine
Swine, Miniature
Wound Healing/*drug effects/genetics
Radiation-Protective Agents
Curcumin
Figure
Reference
-
1. Abraham SK, Sarma L, Kesavan PC. Protective effects of chlorogenic acid, curcumin and β-carotene against γ-radiation-induced in vivo chromosomal damage. Mutat Res. 1993; 303:109–112.
Article2. Arellano A, Santoyo S, Martin C, Ygartua P. Influence of propylene glycol and isopropyl myristate on the in vitro percutaneous penetration of diclofenac sodium from carbopol gels. Eur J Pharm Sci. 1999; 7:129–135.
Article3. Arellano A, Santoyo S, Martn C, Ygartua P. Surfactant effects on the in vitro percutaneous absorption of diclofenac sodium. Eur J Drug Metab Pharmacokinet. 1998; 23:307–112.
Article4. Asai A, Miyazawa T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000; 67:2785–2793.
Article5. Bernatchez SF, Parks PJ, Grussing DM, Matalas SL, Nelson GS. Histological characterization of a delayed wound healing model in pig. Wound Repair Regen. 1998; 6:223–233.
Article6. Bernstein EF, Sullivan FJ, Mitchell JB, Salomon GD, Glatstein E. Biology of chronic radiation effect on tissues and wound healing. Clin Plast Surg. 1993; 20:435–453.
Article7. Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, DiDonato JA, Feinstein E, Gudkov AV. An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008; 320:226–230.
Article8. Denham JW, Hauer-Jensen M, Peters LJ. Is it time for a new formalism to categorize normal tissue radiation injury? Int J Radiat Oncol Biol Phys. 2001; 50:1105–1106.
Article9. Goel A, Jhurani S, Aggarwal BB. Multi-targeted therapy by curcumin: how spicy is it? Mol Nutr Food Res. 2008; 52:1010–1030.
Article10. Hoashi T, Okochi H, Kadono T, Tamaki K, Nishida M, Futami S, Maekawa K. A case of acute radiation syndrome from the dermatological aspect. Br J Dermatol. 2008; 158:597–602.
Article11. Hopewell JW. The skin: its structure and response to ionizing radiation. Int J Radiat Biol. 1990; 57:751–773.
Article12. Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991; 51:813–819.13. Inano H, Onoda M. Radioprotective action of curcumin extracted from Curcuma longa LINN: inhibitory effect on formation of urinary 8-hydroxy-2′-deoxyguanosine, tumorigenesis, but not mortality, induced by γ-ray irradiation. Int J Radiat Oncol Biol Phys. 2002; 53:735–743.
Article14. Jagetia GC. Radioprotective potential of plants and herbs against the effects of ionizing radiation. J Clin Biochem Nutr. 2007; 40:74–81.
Article15. Jagetia GC, Rajanikant GK. Curcumin treatment enhances the repair and regeneration of wounds in mice exposed to hemibody γ-irradiation. Plast Reconstr Surg. 2005; 115:515–528.
Article16. Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009; 14:141–153.17. Kim JS, Yang M, Kim SH, Shin T, Moon C. Neurobiological toxicity of radiation in hippocampal cells. Histol Histopathol. 2013; 28:301–310.18. Kim JS, Rhim KJ, Jang WS, Lee SJ, Son Y, Lee SS, Park S, Lim SM. β-irradiation (166Ho patch)-induced skin injury in the mini-pig: effects on NF-κB and COX-2 expression in the skin. J Vet Sci. 2015; 16:1–9.
Article19. Kim JS, Yang M, Lee CG, Kim SD, Kim JK, Yang K. In vitro and in vivo protective effects of granulocyte colony-stimulating factor against radiation-induced intestinal injury. Arch Pharm Res. 2013; 36:1252–1261.
Article20. Kim SH, Kim SR, Lee HJ, Oh H, Ryu SY, Lee YS, Kim TH, Jo SK. Apoptosis in growing hair follicles following gamma-irradiation and application for the evaluation of radioprotective agents. In Vivo. 2003; 17:211–214.21. Kim SH, Lee HJ, Kim JS, Moon C, Kim JC, Park HR, Jung U, Jang JS, Jo SK. Protective effect of an herbal preparation (HemoHIM) on radiation-induced intestinal injury in mice. J Med Food. 2009; 12:1353–1358.
Article22. Kouvaris J, Kouloulias V, Kokakis J, Matsopoulos G, Myrsini B, Vlahos L. The cytoprotective effect of amifostine in acute radiation dermatitis: a retrospective analysis. Eur J Dermatol. 2002; 12:458–462.23. Liang L, Hu D, Liu W, Williams JP, Okunieff P, Ding I. Celecoxib reduces skin damage after radiation: selective reduction of chemokine and receptor mRNA expression in irradiated skin but not in irradiated mammary tumor. Am J Clin Oncol. 2003; 26:S114–S121.24. Okunieff P, Xu J, Hu D, Liu W, Zhang L, Morrow G, Pentland A, Ryan JL, Ding I. Curcumin protects against radiation-induced acute and chronic cutaneous toxicity in mice and decreases mRNA expression of inflammatory and fibrogenic cytokines. Int J Radiat Oncol Biol Phys. 2006; 65:890–898.
Article25. Panchatcharam M, Miriyala S, Gayathri VS, Suguna L. Curcumin improves wound healing by modulating collagen and decreasing reactive oxygen species. Mol Cell Biochem. 2006; 290:87–96.
Article26. Patel NA, Patel NJ, Patel RP. Formulation and evaluation of curcumin gel for topical application. Pharm Dev Technol. 2009; 14:80–89.
Article27. Phan TT, See P, Lee ST, Chan SY. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001; 51:927–931.
Article28. Ravindranath V, Chandrasekhara N. Absorption and tissue distribution of curcumin in rats. Toxicology. 1980; 16:259–265.
Article29. Ryan JL, Heckler CE, Ling M, Katz A, Williams JP, Pentland AP, Morrow GR. Curcumin for radiation dermatitis: a randomized, double-blind, placebo-controlled clinical trial of thirty breast cancer patients. Radiat Res. 2013; 180:34–43.
Article30. Shah VP, Behl CR, Flynn GL, Higuchi WI, Schaefer H. Principles and criteria in the development and optimization of topical therapeutic products. J Pharm Sci. 1992; 81:1051–1054.
Article31. Sidhu GS, Mani H, Gaddipati JP, Singh AK, Seth P, Banaudha KK, Patnaik GK, Maheshwari RK. Curcumin enhances wound healing in streptozotocin induced diabetic rats and genetically diabetic mice. Wound Repair Regen. 1999; 7:362–374.
Article32. Sidhu GS, Singh AK, Thaloor D, Banaudha KK, Patnaik GK, Srimal RC, Maheshwari RK. Enhancement of wound healing by curcumin in animals. Wound Repair Regen. 1998; 6:167–177.
Article33. Son TG, Gong EJ, Bae MJ, Kim SD, Heo K, Moon C, Yang K, Kim JS. Protective effect of genistein on radiation-induced intestinal injury in tumor bearing mice. BMC Complement Altern Med. 2013; 13:103.
Article34. Sonis ST, O'Donnell KE, Popat R, Bragdon C, Phelan S, Cocks D, Epstein JB. The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol. 2004; 40:170–176.
Article35. Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen. 2001; 9:66–76.
Article36. Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-κB activation. Mutat Res. 2001; 480-481:243–268.
Article37. Swindle MM, Makin A, Herron AJ, Clubb FJ Jr, Frazier KS. Swine as models in biomedical research and toxicology testing. Vet Pathol. 2012; 49:344–356.
Article38. Wang XJ, Lin S, Kang HF, Dai ZJ, Bai MH, Ma XL, Ma XB, Liu MJ, Liu XX, Wang BF. The effect of Rhizoma Coptidis and Coptis chinensis aqueous extract on radiation-induced skin injury in a rat model. BMC Complement Altern Med. 2013; 13:105.39. Yeoh ASJ, Gibson RJ, Yeoh EEK, Bowen JM, Stringer AM, Giam KA, Keefe DMK. A novel animal model to investigate fractionated radiotherapy-induced alimentary mucositis: the role of apoptosis, p53, nuclear factor-κB, COX-1, and COX-2. Mol Cancer Ther. 2007; 6:2319–2327.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antipruritic effect of curcumin on histamine-induced itching in mice
- Therapeutic Effects of Topical Application of Ozone on Acute Cutaneous Wound Healing
- The Effect of Curcumin on Breast Cancer Cells
- Recent Developments in Delivery, Bioavailability, Absorption and Metabolism of Curcumin: the Golden Pigment from Golden Spice
- Establishment of normal reference of radiological morphology of renal artery in mini-pigs by renal angiography