Allergy Asthma Respir Dis.
2018 May;6(3):141-148. 10.4168/aard.2018.6.3.141.
Precision medicine for the best treatment of chronic obstructive airway disease
- Affiliations
-
- 1Department of Internal Medicine, Asan Medical Center, Seoul, Korea. yscho@amc.seoul.kr
- KMID: 2412516
- DOI: http://doi.org/10.4168/aard.2018.6.3.141
Abstract
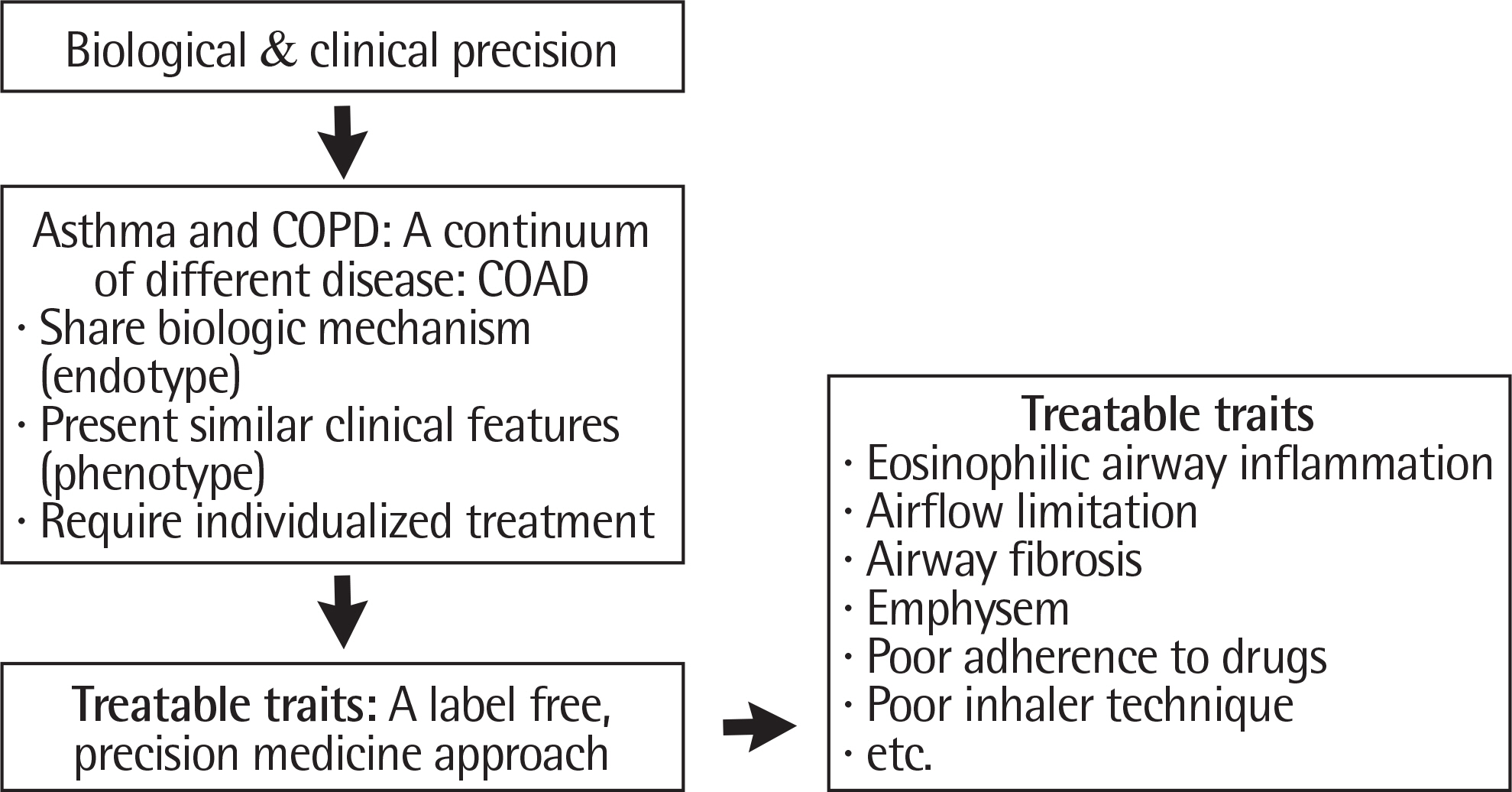
- Bronchial asthma and chronic obstructive pulmonary disease (COPD) are 2 representative diseases of chronic obstructive inflammatory airway diseases, and both show a wide range of heterogeneity in their clinical features. Although one end of typical asthma and the other end of COPD are clearly different, both diseases share lots of similarities in biological aspects and clinical manifestations. Currently, 2 different guidelines exist for asthma and COPD management, respectively, and in many clinical situations it is not easy to manage patients especially who have both features and show refractoriness to available medications. Since the features of the diseases are remarkably diverse in terms of clinical courses, prognosis and responses to therapeutic drugs, there have been vigorous efforts to classify appropriate subtypes in order to improve management of the diseases. However, dichotomous thinking about asthma and COPD precludes precise classification of the diseases in the real world. In this article, thus, chronic obstructive airway disease (COAD) ranging from asthma particularly in adults to COPD is proposed as 1 target subject to analyze precise classification based on exact phenotyping and endotyping of the diseases. In the current article, the reasonable precision medicine approach is also suggested based on treatable traits of COAD to achieve the best treatment for COAD patients.
Keyword
MeSH Terms
Figure
Reference
-
1. The Global Initiative for Asthma. 2017 GINA report, global strategy for asthma management and prevention [Internet]. Global Initiative for Asthma;c2017. [cited 2017 Oct 7]. Available from. http://ginasthma.org/gina-reports/.2. Reid L. The role of chronic bronchitis in the production of “chronic obstructive pulmonary emphysema”. J Am Med Womens Assoc. 1965; 20:633–8.3. Orie NG, Sluiter HJ, De Vries K, Tammeling GJ, Witkop J. The host factor in bronchitis. Orie NG, Sluiter HJ, editors. Bronchitis. Assen (The Neth-erlands): Royal Van Gorcum;1961.4. Cosío BG, Pérez de Llano L, Lopez Viña A, Torrego A, Lopez-Campos JL, Soriano JB, et al. Th-2 signature in chronic airway diseases: towards the extinction of asthma-COPD overlap syndrome? Eur Respir J. 2017; 49:pii: 1602397.
Article5. Kim TB, Oh YM, Chang YS, Cho YS, Jang AS, Cho SH, et al. The reality of an intermediate type between asthma and COPD in practice. Respir Care. 2012; 57:1248–53.
Article6. Wheaton AG, Pleasants RA, Croft JB, Ohar JA, Heidari K, Mannino DM, et al. Gender and asthma-chronic obstructive pulmonary disease overlap syndrome. J Asthma. 2016; 53:720–31.
Article7. Gibson PG. Inflammatory phenotypes in adult asthma: clinical applica-tions. Clin Respir J. 2009; 3:198–206.
Article8. Simpson JL, Scott R, Boyle MJ, Gibson PG. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 2006; 11:54–61.
Article9. Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, et al. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014; 133:1557–63.e5.
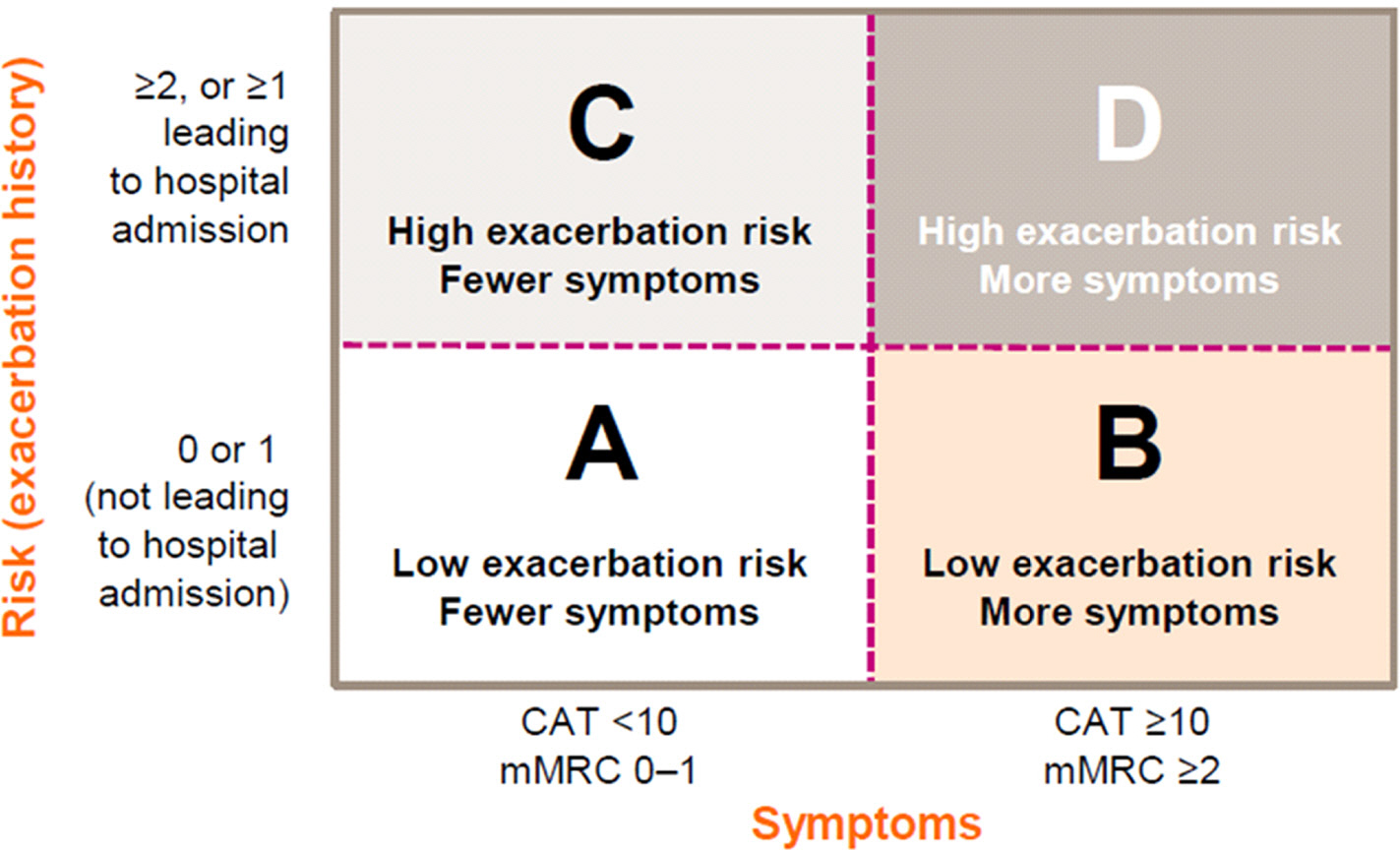
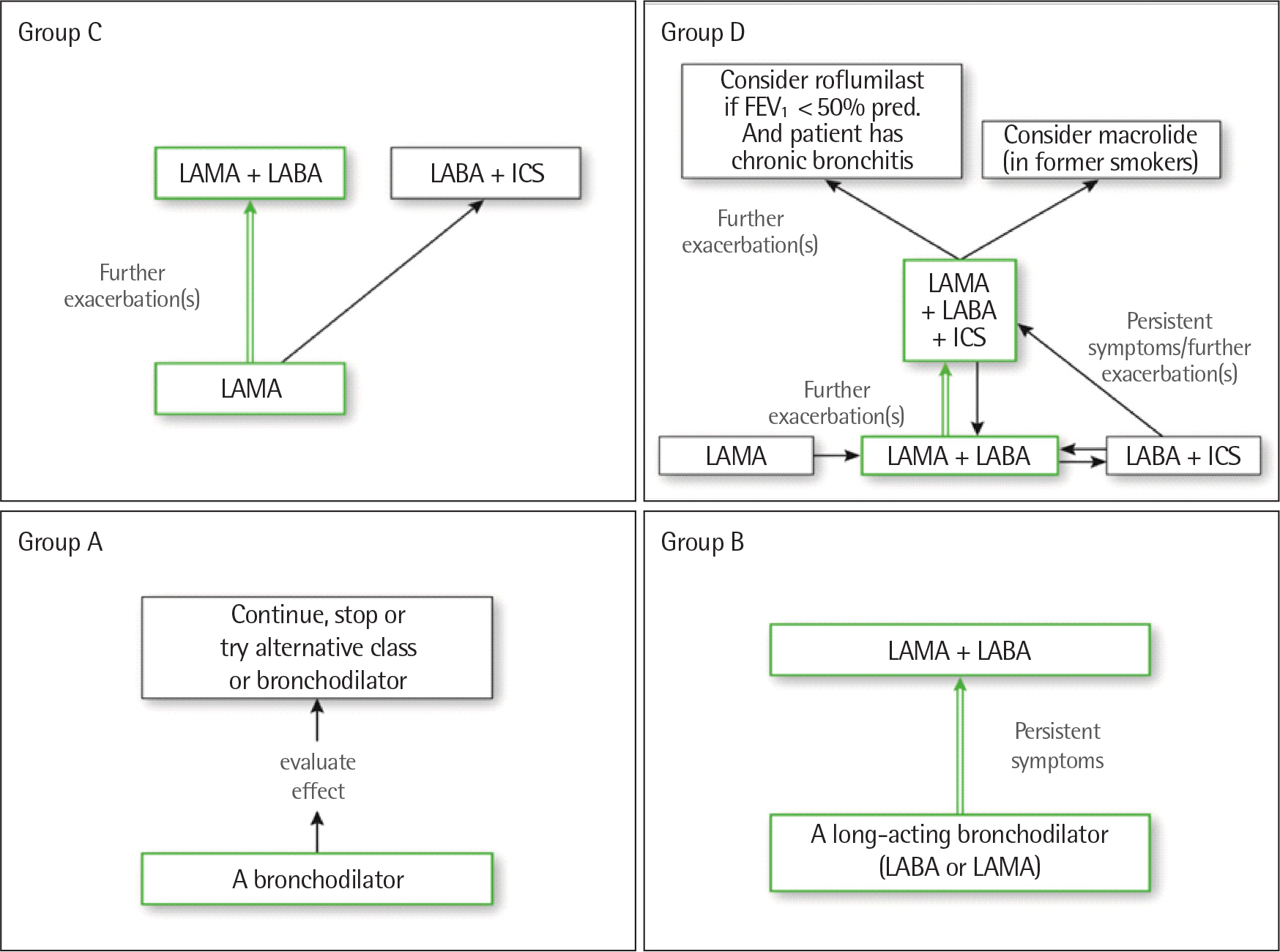
Article10. Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 global strategy for the diagnosis, management and prevention of COPD [Internet]. Global Initiative for Chronic Obstructive Lung Disease;c2018. [cited 2017 Oct 8]. Available from. www.goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/.11. Wenzel S. Severe asthma in adults. Am J Respir Crit Care Med. 2005; 172:149–60.
Article12. Balzar S, Wenzel SE, Chu HW. Transbronchial biopsy as a tool to evaluate small airways in asthma. Eur Respir J. 2002; 20:254–9.
Article13. Elias JA. Airway remodeling in asthma. Unanswered questions. Am J Respir Crit Care Med. 2000; 161(3 Pt 2):S168–71.14. Boulet L, Bélanger M, Carrier G. Airway responsiveness and bronchial-wall thickness in asthma with or without fixed airflow obstruction. Am J Respir Crit Care Med. 1995; 152:865–71.
Article15. Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003; 167:1360–8.
Article16. Busse WW, Banks-Schlegel S, Wenzel SE. Pathophysiology of severe asthma. J Allergy Clin Immunol. 2000; 106:1033–42.
Article17. Kim TB, Jang AS, Kwon HS, Park JS, Chang YS, Cho SH, et al. Identification of asthma clusters in two independent Korean adult asthma cohorts. Eur Respir J. 2013; 41:1308–14.
Article18. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010; 181:315–23.
Article19. Hinks TS, Brown T, Lau LC, Rupani H, Barber C, Elliott S, et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol. 2016; 138:61–75.
Article20. Kuo CS, Pavlidis S, Loza M, Baribaud F, Rowe A, Pandis I, et al. T-helper cell type 2 (Th2) and non-Th2 molecular phenotypes of asthma using sputum transcriptomics in U-BIOPRED. Eur Respir J. 2017; 49:pii: 1602135.
Article21. Marichal T, Mesnil C, Bureau F. Homeostatic eosinophils: characteristics and functions. Front Med (Lausanne). 2017; 4:101.
Article22. Rothenberg ME. A hidden residential cell in the lung. J Clin Invest. 2016; 126:3185–7.
Article23. Woodruff PG, Agusti A, Roche N, Singh D, Martinez FJ. Current concepts in targeting chronic obstructive pulmonary disease pharmacother-apy: making progress towards personalised management. Lancet. 2015; 385:1789–98.
Article24. Bafadhel M, McCormick M, Saha S, McKenna S, Shelley M, Hargadon B, et al. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration. 2012; 83:36–44.
Article25. Ghebre MA, Bafadhel M, Desai D, Cohen SE, Newbold P, Rapley L, et al. Biological clustering supports both "Dutch" and "British" hypotheses of asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2015; 135:63–72.
Article26. Bardin PG, Price D, Chanez P, Humbert M, Bourdin A. Managing asthma in the era of biological therapies. Lancet Respir Med. 2017; 5:376–8.
Article27. Scanlon ST, McKenzie AN. Type 2 innate lymphoid cells: new players in asthma and allergy. Curr Opin Immunol. 2012; 24:707–12.
Article28. Sears MR. Safe use of long-acting β-agonists: what have we learnt? Expert Opin Drug Saf. 2011; 10:767–78.
Article29. Park HW, Yang MS, Park CS, Kim TB, Moon HB, Min KU, et al. Additive role of tiotropium in severe asthmatics and Arg16Gly in ADRB2 as a potential marker to predict response. Allergy. 2009; 64:778–83.30. Thomas M, Halpin DM, Miravitlles M. When is dual bronchodilation indicated in COPD? Int J Chron Obstruct Pulmon Dis. 2017; 12:2291–305.
Article31. Cho YS. Effective strategies for managing asthma exacerbations for precision medicine. Allergy Asthma Immunol Res. 2017; 9:463–5.
Article32. Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate S, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016; 47:410–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pathogenesis and pathophysiology of COPD
- Chronic Obstructive Pulmonary Disease and the Airway Microbiome: What Respirologists Need to Know
- Chronic Obstructive Pulmonary Disease: Respiratory Review of 2013
- Evaluation of airway resistance in normal subjects and patients with chronic obstructive pulmonary disease
- Small Airway Disease in Patients with Chronic Obstructive Pulmonary Disease