Transl Clin Pharmacol.
2017 Mar;25(1):15-20. 10.12793/tcp.2017.25.1.15.
Enhancement of skin permeation of vitamin C using vibrating microneedles
- Affiliations
-
- 1College of Pharmacy and Institute of Drug Research & Development, Chungnam National University, Daejeon 34134, South Korea. chocw@cnu.ac.kr
- 2Department of Aeronautical Engineering, Hanseo University, 236-49 Gomseom-ro, Nam-myun, Taean-Gun, Chungnam-do 32158, South Korea.
- 3Korea Institute of Industrial Technology, 1278-18 sa 1-dong, Sangnok-gu, Ansan, Gyeonggi 15585, South Korea.
- KMID: 2411414
- DOI: http://doi.org/10.12793/tcp.2017.25.1.15
Abstract
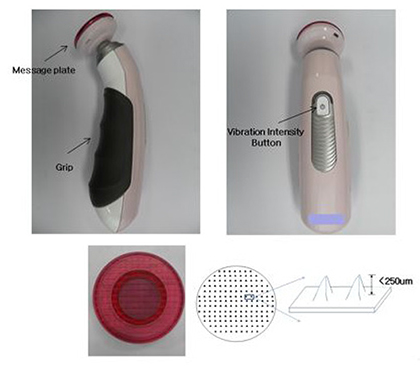
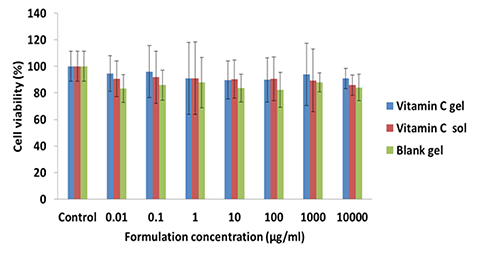
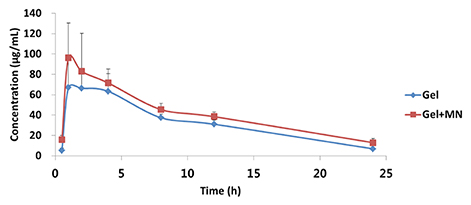
- This study was performed to evaluate the use of vibrating microneedles for the transdermal delivery of vitamin C. The microneedles were designed to vibrate at three levels of intensity. In vitro permeation by vitamin C was evaluated according to the specific conditions such as vibration intensity (levels 1, 2 and 3), application time (1, 3, 5, 7 and 10 min), and application power (500, 700 and 1,000 g). The highest permeation of vitamin C was observed at level 3 of vibration intensity, 5 min of application, and 1,000 g of application power. Vitamin C gel showed no cytotoxic effect against Pam212 cells or skin irritation effects. A pharmacokinetic study of the gel in rats was conducted under optimized conditions. The AUC₀-∞ and C(max) increased 1.35-fold and 1.44-fold, respectively, compared with those after vitamin C gel without application with vibrating microneedles. The present study suggests that vibrating microneedles can be used to facilitate the skin permeability of vitamin C under optimal conditions.
MeSH Terms
Figure
Reference
-
1. Naik A, Kalia YN, Guy RH. Transdermal drug delivery: overcoming the skin's barrier function. Pharm Sci Technolo Today. 2000; 3:318–326.
Article2. Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008; 26:1261–1268.
Article3. Kirjavainen M, Mönkkönen J, Saukkosaari M, Valjakka-Koskela R, Kiesvaara J, Urtti A. Phospholipids affect stratum corneum lipid bilayer fluidity and drug partitioning into the bilayers. J Control Release. 1999; 58:207–214.
Article4. Parsaee S, Sarbolouki MN, Parnianpour M. In-vitro release of diclofenac diethylammonium from lipid-based formulations. Int J Pharm. 2002; 241:185–190.
Article5. Ho HO, Huang FC, Sokoloski TD, Sheu MT. The influence of cosolvents on the in-vitro percutaneous penetration of diclofenac sodium from a gel system. J Pharm Pharmacol. 1994; 46:636–642.
Article6. Shin SC, Kim HJ, Oh IJ, Cho CW, Yang KH. Development of tretinoin gels for enhanced transdermal delivery. Eur J Pharm Biopharm. 2005; 60:67–71.
Article7. Henry S, McAllister DV, Allen MG, Prausnitz MR. Microfabricated microneedles: a novel approach to transdermal drug delivery. J Pharm Sci. 1998; 87:922–925.
Article8. Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Control Release. 2007; 117:227–237.
Article9. Andrianov AK, DeCollibus DP, Gillis HA, Kha HH, Marin A, Prausnitz MR, et al. Poly [di (carboxylatophenoxy) phosphazene] is a potent adjuvant for intradermal immunization. Proc Natl Acad Sci USA. 2009; 106:18936–18941. DOI: 10.1073/pnas.0908842106.
Article10. Lee JW, Park JH, Prausnitz MR. Dissolving microneedles for transdermal drug delivery. Biomaterials. 2008; 29:2113–2124. DOI: 10.1016/j.biomaterials.2007.12.048.
Article11. Ito Y, Hagiwara E, Saeki A, Sugioka N, Takada K. Feasibility of microneedles for percutaneous absorption of insulin. Eur J Pharm Sci. 2006; 29:82–88.
Article12. Sivamani RK, Stoebe B, Wu GC, Zhai H, Liepmann D, Maibach H. Clinical microneedle injection of methyl nicotinate: stratum corneum penetration. Skin Res Technol. 2005; 11:152–156.
Article13. Gupta J, Felner EI, Prausnitz MR. Minimally invasive insulin delivery in subjects with type 1 diabetes using hollow microneedles. Diabetes Technol Ther. 2009; 11:329–337. DOI: 10.1089/dia.2008.0103.
Article14. Mikszta JA, Laurent PE. Cutaneous delivery of prophylactic and therapeutic vaccines: historical perspective and future outlook. Expert Rev Vaccines. 2008; 7:1329–1339. DOI: 10.1586/14760584.7.9.1329.
Article15. Machlin LJ. Handbook of Vitamins. 2nd Ed. Marcel Dekker, Inc;1991.16. Bossi A, Piletsky SA, Piletska EV, Righetti PG, Turner AP. An assay for ascorbic acid based on polyaniline-coated microplates. Anal Chem. 2000; 72:4296–4300.
Article17. Yamamoto I, Tai A, Fujinami Y, Sasaki K, Okazaki S. Synthesis and characterization of a series of novel monoacylated ascorbic acid derivatives, 6-O-acyl-2-O-α-Dglucopyranosyl- l-ascorbic acids, as skin antioxidants. J Med Chem. 2002; 45:462–468.18. Tsuchiya H, Bates CJ. Changes in collagen cross-link ratios in bone and urine of guinea pigs fed graded dietary vitamin C: A functional index of vitamin C status. J Nutr Biochem. 1998; 9:402–407.
Article19. Horino Y, Takahashi S, Miura T, Takahashi Y. Prolonged hypoxia accelerates the posttranscriptional process of collagen synthesis in cultured fibroblasts. Life Sci. 2002; 71:3031–3045.
Article20. Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001; 14:101–114.
Article21. Lombry C, Dujardin N, Préat V. Transdermal delivery of macromolecules using skin electroporation. Pharm Res. 2000; 17:32–37.22. Gallarate M, Carlotti ME, Trotta M, Bovo S. On the stability of ascorbic acid in emulsified systems for topical and cosmetic use. Int J Pharm. 1999; 188:233–241.
Article23. Austria R, Semenzato A, Bettero A. Stability of vitamin C derivatives in solution and topical fornmlations. J Pharm Biomed Anal. 1997; 15:795–801.24. Spiclin P, Gasperlin M, Kmetec V. Stability of ascorbyl palmitate in topical microemulsions. Int J Pharm. 2001; 222:271–279.
Article25. Semenzato A, Austria R, Dall'Aglio C, Bettero A. High-performance liquid chromatographic determination of ionic compounds in cosmetic emulsions: application to magnesium ascorbyl phosphate. J Chromatogr A. 1995; 705:385–389.
Article26. Alexander A, Dwivedi S, Ajazuddin , Giri TK, Saraf S, Saraf S, et al. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J Control Release. 2012; 164:26–40. DOI: 10.1016/j.jconrel.2012.09.017.
Article27. Yong Z, Matthew PS, Russell JM, Michael J. Transdermal prodrug delivery for radionuclide decorporation: nonaqueous gel formulation development and in vitro and in vivo assessment. Drug Dev Res. 2013; 74:322–331.
Article28. Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, et al. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004; 97:503–511.
Article29. Donnelly RF, Garland MJ, Morrow DI, Migalska K, Singh TR, Majithiya R, et al. Optical coherence tomography is a valuable tool in the study of the effects of microneedle geometry on skin penetration characteristics and in-skin dissolution. J Control Release. 2010; 147:333–341. DOI: 10.1016/j.jconrel.2010.08.008.
Article30. Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012; 64:1547–1568. DOI: 10.1016/j.addr.2012.04.005.
Article31. Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011; 154:148–155. DOI: 10.1016/j.jconrel.2011.05.021.
Article32. Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci USA. 2008; 105:2058–2063. DOI: 10.1073/pnas.0710355105.
Article33. Kalluri H, Kolli CS, Banga AK. Characterization of microchannels created by metal microneedles: formation and closure. AAPS J. 2011; 13:473–481. DOI: 10.1208/s12248-011-9288-3.
Article34. Yang JH, Lee SY, Han YS, Park KC, Choy JH. Efficient Transdermal Penetration and Improved Stability of L-Ascorbic Acid Encapsulated in an Inorganic Nanocapsule. Bull Korean Chem Soc. 2003; 24:499–503.
Article