J Rheum Dis.
2018 Apr;25(2):131-139. 10.4078/jrd.2018.25.2.131.
Hemoglobin A1c, Not Glycated Albumin, Can Independently Reflect the Ankylosing Spondylitis Disease Activity Score
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. sangwonlee@yuhs.ac
- KMID: 2408931
- DOI: http://doi.org/10.4078/jrd.2018.25.2.131
Abstract
OBJECTIVE
This study examined whether glycated hemoglobin (HbA1c) and glycated albumin (GA) are well correlated with the Ankylosing Spondylitis Disease Activity Score (ASDAS)-erythrocyte sedimentation rate (ESR), and ASDAS-C-reactive protein (CRP) in AS patients without medical conditions affecting the glycated protein levels.
METHODS
The data of 76 patients with AS were analyzed. Univariate and multivariate analyses of the variables associated with ASDAS-ESR and ASDAS-CRP were performed using a linear regression test. The patients were divided into active and inactive AS groups based on an ASDAS-CRP of 2.1, and the variables between the two groups were compared.
RESULTS
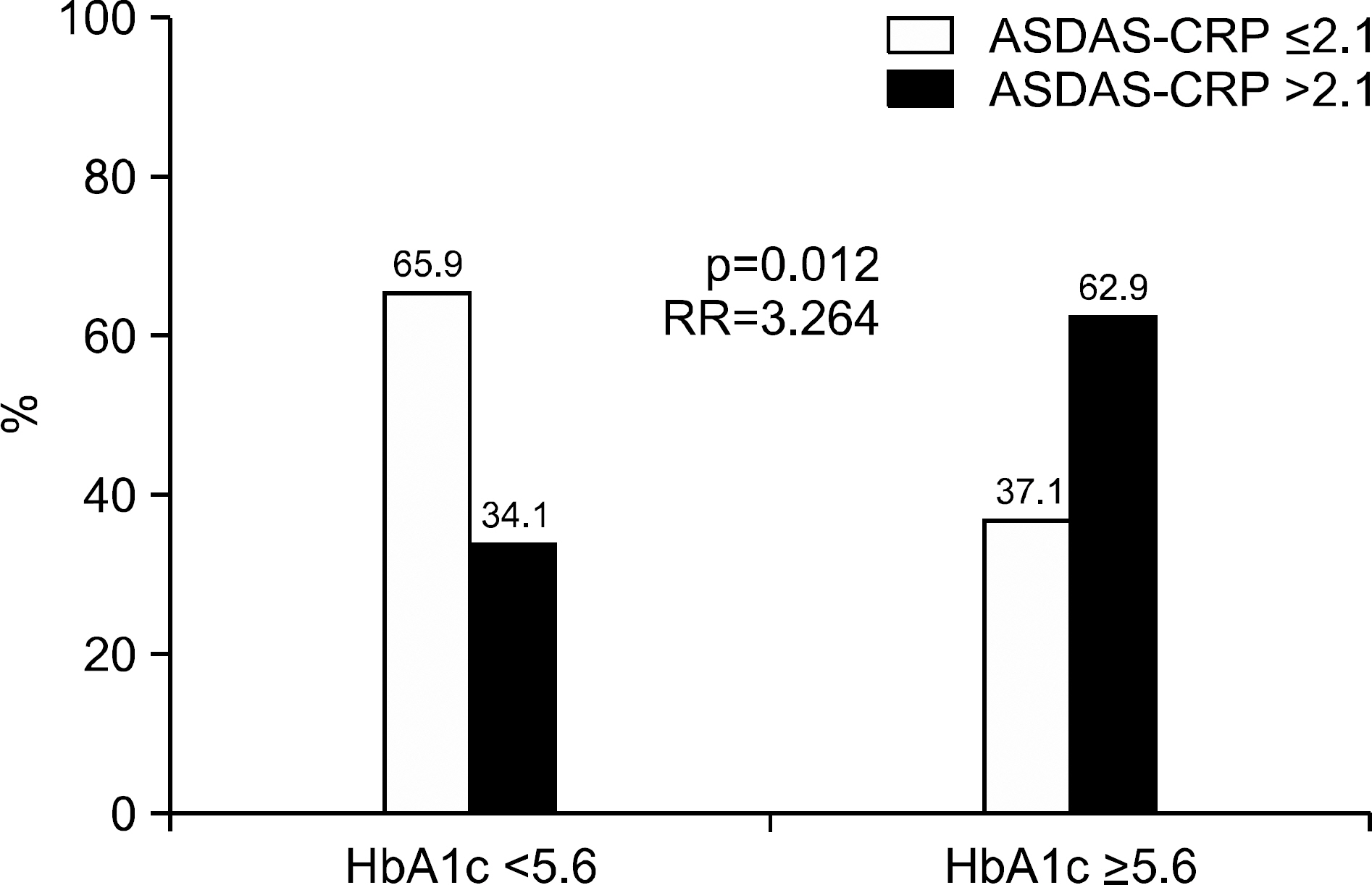
ASDAS-ESR did not correlated with either HbA1c or GA. ASDAS-CRP was positively correlated with HbA1c (r=0.315, p=0.006) and the white blood cell (r=0.288, p=0.012), and inversely correlated with hemoglobin (r=−0.241, p=0.036) and serum albumin (r=−0.262, p=0.022), but not GA. Multivariate analysis revealed HbA1c and white blood cell to be significantly correlated with ASDAS-CRP (β=0.234, p=0.033 and β=0.265, p=0.017). The mean HbA1c, not GA, of the active group was significantly higher than that of the inactive group (p=0.020). In addition, the optimal cut-off value of HbA1c was set to 5.6, and the patients with HbA1c ≥5.6 were found to have a 3.3 times higher risk of active AS than those without.
CONCLUSION
HbA1c was significantly correlated with ASDAS-CRP, and could be a useful marker to reflect ASDAS-CRP in AS patients without medical conditions affecting the glycated protein levels.
MeSH Terms
Figure
Reference
-
1. Pedersen SJ, Sørensen IJ, Garnero P, Johansen JS, Madsen OR, Tvede N, et al. ASDAS, BASDAI and different treatment responses and their relation to biomarkers of inflammation, cartilage and bone turnover in patients with axial spondyloarthritis treated with TNFα inhibitors. Ann Rheum Dis. 2011; 70:1375–81.
Article2. Braun J, Davis J, Dougados M, Sieper J, van der Linden S, van der Heijde D. First update of the international ASAS consensus statement for the use of anti-TNF agents in patients with ankylosing spondylitis. Ann Rheum Dis. 2006; 65:316–20.
Article3. Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E, et al. 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2011; 70:896–904.
Article4. Dougados M, Gueguen A, Nakache JP, Velicitat P, Zeidler H, Veys E, et al. Clinical relevance of C-reactive protein in axial involvement of ankylosing spondylitis. J Rheumatol. 1999; 26:971–4.5. Garrett S, Jenkinson T, Kennedy LG, Whitelock H, Gaisford P, Calin A. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol. 1994; 21:2286–91.6. van der Heijde D, Lie E, Kvien TK, Sieper J, Van den Bosch F, Listing J, et al. ASDAS, a highly discriminatory ASAS-en-dorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis. 2009; 68:1811–8.
Article7. Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, et al. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: a mechanism for amplification of inflammatory responses. Circulation. 2002; 105:816–22.8. Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006; 114:597–605.9. Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J. 2010; 57:751–62.
Article10. Koga M, Murai J, Morita S, Saito H, Kasayama S. Comparison of annual variability in HbA1c and glycated albumin in patients with type 1 vs. type 2 diabetes mellitus. J Diabetes Complications. 2013; 27:211–3.
Article11. Koga M, Otsuki M, Matsumoto S, Saito H, Mukai M, Kasayama S. Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta. 2007; 378:48–52.
Article12. Park JS, Song J, Park YB, Lee SK, Lee SW. Glycated albumin increases with disease activity in rheumatoid factor positive rheumatoid arthritis patients with normal fasting glucose and HbA1c. Joint Bone Spine. 2017; 84:115–8.
Article13. van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984; 27:361–8.14. Koga M, Murai J, Saito H, Kasayama S, Imagawa A, Hanafusa T, et al. Serum glycated albumin to haemoglobin A(1C) ratio can distinguish fulminant type 1 diabetes mellitus from type 2 diabetes mellitus. Ann Clin Biochem. 2010; 47:313–7.
Article15. Koga M, Murai J, Saito H, Matsumoto S, Kasayama S. Effects of thyroid hormone on serum glycated albumin levels: study on non-diabetic subjects. Diabetes Res Clin Pract. 2009; 84:163–7.
Article16. Okada T, Nakao T, Matsumoto H, Nagaoka Y, Tomaru R, Iwasawa H, et al. Influence of proteinuria on glycated albumin values in diabetic patients with chronic kidney disease. Intern Med. 2011; 50:23–9.
Article17. Nomura Y, Nanjo K, Miyano M, Kikuoka H, Kuriyama S, Maeda M, et al. Hemoglobin A1 in cirrhosis of the liver. Diabetes Res. 1989; 11:177–80.18. Panzer S, Kronik G, Lechner K, Bettelheim P, Neumann E, Dudczak R. Glycosylated hemoglobins (GHb): an index of red cell survival. Blood. 1982; 59:1348–50.
Article19. Machado P, Navarro-Compán V, Landewé R, van Gaalen FA, Roux C, van der Heijde D. Calculating the ankylosing spondylitis disease activity score if the conventional c-reactive protein level is below the limit of detection or if high-sensitivity c-reactive protein is used: an analysis in the DESIR cohort. Arthritis Rheumatol. 2015; 67:408–13.20. Calin A, Garrett S, Whitelock H, Kennedy LG, O'Hea J, Mallorie P, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994; 21:2281–5.21. Jones SD, Steiner A, Garrett SL, Calin A. The bath ankylosing spondylitis patient global score (BAS-G). Br J Rheumatol. 1996; 35:66–71.
Article22. Pu LJ, Lu L, Xu XW, Zhang RY, Zhang Q, Zhang JS, et al. Value of serum glycated albumin and high-sensitivity C-reactive protein levels in the prediction of presence of coronary artery disease in patients with type 2 diabetes. Cardiovasc Diabetol. 2006; 5:27.
Article23. Maria Z, Yin W, Rubenstein DA. Combined effects of physiologically relevant disturbed wall shear stress and glycated albumin on endothelial cell functions associated with inflammation, thrombosis and cytoskeletal dynamics. J Diabetes Investig. 2014; 5:372–81.
Article24. Gustavsson CG, Agardh CD. Markers of inflammation in patients with coronary artery disease are also associated with glycosylated haemoglobin A1c within the normal range. Eur Heart J. 2004; 25:2120–4.25. Rudwaleit M, Haibel H, Baraliakos X, Listing J, Märker-Hermann E, Zeidler H, et al. The early disease stage in axial spondylarthritis: results from the German Spondyloarthritis Inception Cohort. Arthritis Rheum. 2009; 60:717–27.
Article26. Kim KJ, Lee BW. The roles of glycated albumin as intermediate glycation index and pathogenic protein. Diabetes Metab J. 2012; 36:98–107.
Article27. Danve A, O'Dell J. The ongoing quest for biomarkers in Ankylosing Spondylitis. Int J Rheum Dis. 2015; 18:826–34.
Article28. de Rotte MC, de Jong PH, den Boer E, Pluijm SM, Özcan B, Weel AE, et al. Effect of methotrexate use and erythrocyte methotrexate polyglutamate on glycosylated hemoglobin in rheumatoid arthritis. Arthritis Rheumatol. 2014; 66:2026–36.
Article29. Krogh Jensen M, Ekelund S, Svendsen L. Folate and homocysteine status and haemolysis in patients treated with sul-phasalazine for arthritis. Scand J Clin Lab Invest. 1996; 56:421–9.30. Lithell HO. Effect of antihypertensive drugs on insulin, glucose, and lipid metabolism. Diabetes Care. 1991; 14:203–9.
Article31. Goyal A, Singh S, Tandon N, Gupta N, Gupta YK. Effect of atorvastatin on pancreatic Beta-cell function and insulin resistance in type 2 diabetes mellitus patients: a randomized pilot study. Can J Diabetes. 2014; 38:466–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Roles of Glycated Albumin as Intermediate Glycation Index and Pathogenic Protein
- The Glycated Albumin to Glycated Hemoglobin Ratio Might Not Be Associated with Carotid Atherosclerosis in Patients with Type 1 Diabetes
- Platelet Distribution Width and Mean Platelet Volume Are Not Correlated with the Disease Activity Indices of Ankylosing Spondylitis
- Bath Ankylosing Spondylitis Disease Activity Index is Associated With the Quality of Sleep in Ankylosing Spondylitis Patients
- Clinieal Values of Single Photon Emission Computed Tomography ( SPECT ) in Ankylosing Spondylitis