Ann Lab Med.
2018 Jul;38(4):375-377. 10.3343/alm.2018.38.4.375.
Necessity of Reticulocyte Calibration for More Accurate and Precise Results
- Affiliations
-
- 1Department of Laboratory Medicine, Chung-Ang University College of Medicine, Seoul, Korea. hyekim@cau.ac.kr
- 2Samkwang Medical Laboratories, Seoul, Korea.
- KMID: 2408079
- DOI: http://doi.org/10.3343/alm.2018.38.4.375
Abstract
- No abstract available.
MeSH Terms
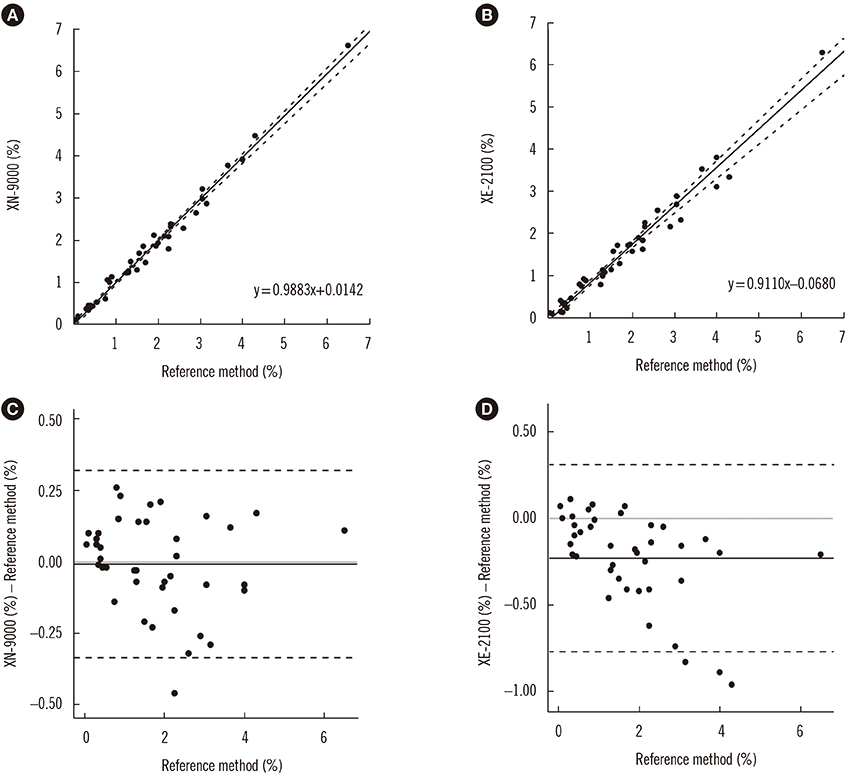
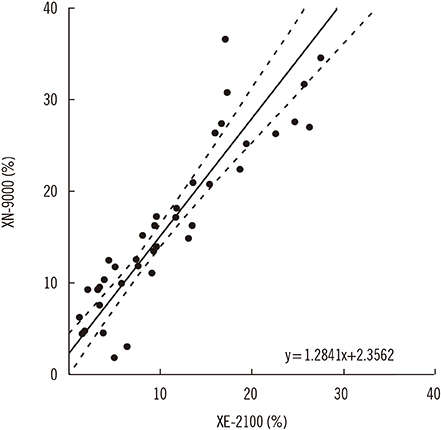
Figure
Reference
-
1. Chang CC, Kass L. Clinical significance of immature reticulocyte fraction determined by automated reticulocyte counting. Am J Clin Pathol. 1997; 108:69–73.
Article2. WADA. Blood analytical requirements for the athlete biological passport. World anti-doping agency. Athlete biological passport operating guidelines and compilation of required elements. V5.0. World Anti-Doping Agency;2014. p. 31–34.3. Sottas PE, Robinson N, Giraud S, Taroni F, Kamber M, Mangin P, et al. Statistical classification of abnormal blood profiles in athletes. Int J Biostat. 2006; 2:1–21.
Article4. Briggs C, Longair I, Kumar P, Singh D, Machin SJ. Performance evaluation of the Sysmex haematology XN modular system. J Clin Pathol. 2012; 65:1024–1030.
Article5. Briggs C, Culp N, Davis B, d'Onofrio G, Zini G, Machin SJ. ICSH guidelines for the evaluation of blood cell analysers including those used for differential leucocyte and reticulocyte counting. Int J Lab Hematol. 2014; 36:613–627.6. CLSI. Methods for Reticulocyte Counting (Automated Blood Cell Counters, Flow Cytometry, and Supravital Dyes). 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2004. CLSI supplement EP44-A2.7. CLSI. Evaluation of Precision of Quantitative Measurement Procedures. 3rd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2014. CLSI supplement EP05-A3.8. Burtis CA, Ashwood ER, Bruns DE, Tietz NW. Tietz textbook of clinical chemistry and molecular diagnostics. 5th ed. St. Louis, Mo.: Saunders;2013.9. Briggs C, Grant D, Machin SJ. Comparison of the automated reticulocyte counts and immature reticulocyte fraction measurements obtained with the ABX Pentra 120 Retic blood analyzer and the Sysmex XE-2100 automated hematology analyzer. Lab Hematol. 2001; 7:75–80.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Significance of Reticulocyte Maturation Parameters in the Differential Diagnosis of Macrocytic Anemias
- The irtQ R package: a user-friendly tool for item response theory-based test data analysis and calibration
- An Evaluation of automated reticulocyte counter R-3000
- Reference Intervals of Reticulocyte Parameters using ADVIA 120
- Reference Intervals for Research Parameters of Reticulocyte Hemoglobin and Platelet Clumps in Healthy Korean Adults