J Korean Soc Radiol.
2017 Sep;77(3):148-156. 10.3348/jksr.2017.77.3.148.
Alterations of White Matter Integrity in Patients with Chronic Obstructive Pulmonary Disease: Tract-Based Analysis Using TRActs Constrained by UnderLying Anatomy
- Affiliations
-
- 1Department of Radiology, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea. samskim@kangwon.ac.kr
- 2Department of Neurology, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea.
- 3Neuroscience Research Institute, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea.
- 4Department of Internal Medicine, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea.
- 5Environmental Health Center, Kangwon National University Hospital, Kangwon National University School of Medicine, Chuncheon, Korea.
- 6Brain Convergence Research Center, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- 7Department of Physical Medicine and Rehabilitation, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea.
- KMID: 2405729
- DOI: http://doi.org/10.3348/jksr.2017.77.3.148
Abstract
- PURPOSE
To investigate structural brain changes and their relationship with cognitive function by determining the alterations of white matter integrity and changes in hippocampal volume that occur during chronic obstructive pulmonary disease (COPD).
MATERIALS AND METHODS
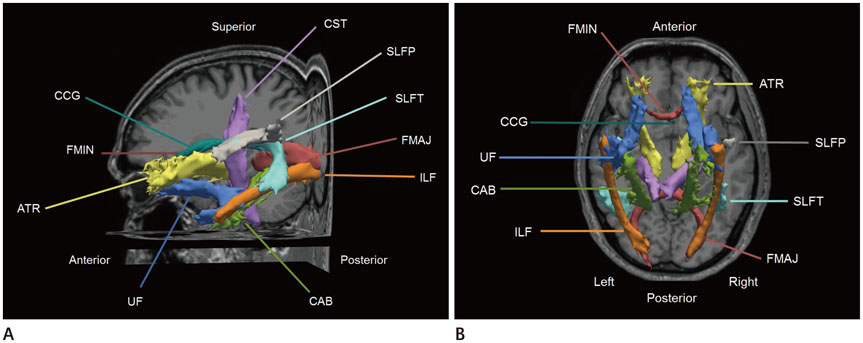
Diffusion tensor images and 3D-T1 MR images were acquired in 13 male nonhypoxemic COPD patients and 13 age- and gender-matched healthy controls. Global probabilistic tractography was used to assess a total of 18 major tracts. We examined the association between the hippocampal volume and diffusivity parameters of white matter tracts.
RESULTS
A significant difference in diffusion parameters between groups was identified for 11 white matter bundles (p < 0.05). No association was demonstrated between the normalized hippocampus volume and diffusion parameters among the participants. Significant associations between Korean version of the Mini-Mental State Examination (K-MMSE) scores and diffusivities were found for six tracts (p < 0.05). Of note, the mean diffusivity (MD) and redial diffusivity (RD) of the left superior longitudinal fasciculus parietal segment showed a significant negative correlation with the K-MMSE score (MD: r = −0.623, p = 0.001; RD: r = −0.408, p = 0.048).
CONCLUSION
Altered white matter integrity was demonstrated across regional white matter bundles, including the superior longitudinal fasciculus, in COPD patients, and it was related to cognitive function. Changes in the volume of the hippocampus should be investigated in further studies.
MeSH Terms
Figure
Reference
-
1. Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform. 2011; 5:23.2. Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002; 17:1429–1436.3. Wouters EF, Creutzberg EC, Schols AM. Systemic effects in COPD. Chest. 2002; 121:5 Suppl. 127S–130S.4. Dodd JW, Chung AW, van den Broek MD, Barrick TR, Charlton RA, Jones PW. Brain structure and function in chronic obstructive pulmonary disease: a multimodal cranial magnetic resonance imaging study. Am J Respir Crit Care Med. 2012; 186:240–245.5. Li J, Fei GH. The unique alterations of hippocampus and cognitive impairment in chronic obstructive pulmonary disease. Respir Res. 2013; 14:140.6. Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006; 51:527–539.7. Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994; 66:259–267.8. Basser PJ. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995; 8:333–344.9. Lee SH, Coutu JP, Wilkens P, Yendiki A, Rosas HD, Salat DH. Alzheimer's disease Neuroimaging Initiative (ADNI). Tract-based analysis of white matter degeneration in Alzheimer's disease. Neuroscience. 2015; 301:79–89.10. Chong CD, Schwedt TJ. Migraine affects white-matter tract integrity: a diffusion-tensor imaging study. Cephalalgia. 2015; 35:1162–1171.11. Sarica A, Cerasa A, Vasta R, Perrotta P, Valentino P, Mangone G, et al. Tractography in amyotrophic lateral sclerosis using a novel probabilistic tool: a study with tract-based reconstruction compared to voxel-based approach. J Neurosci Methods. 2014; 224:79–87.12. Wozniak JR, Mueller BA, Lim KO, Hemmy LS, Day JW. Tractography reveals diffuse white matter abnormalities in myotonic dystrophy type 1. J Neurol Sci. 2014; 341:73–78.13. Storsve AB, Fjell AM, Yendiki A, Walhovd KB. Longitudinal changes in white matter tract integrity across the adult lifespan and its relation to cortical thinning. PLoS One. 2016; 11:e0156770.14. Pfuhl G, King JA, Geisler D, Roschinski B, Ritschel F, Seidel M, et al. Preserved white matter microstructure in young patients with anorexia nervosa? Hum Brain Mapp. 2016; 37:4069–4083.15. Christodoulou JA, Murtagh J, Cyr A, Perrachione TK, Chang P, Halverson K, et al. Relation of white-matter microstructure to reading ability and disability in beginning readers. Neuropsychology. 2016; 03. 07. DOI: 10.1037/neu0000243. [Epub].16. Incalzi RA, Gemma A, Marra C, Muzzolon R, Capparella O, Carbonin P. Chronic obstructive pulmonary disease. An original model of cognitive decline. Am Rev Respir Dis. 1993; 148:418–424.17. Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J. 2010; 35:913–922.18. Ortapamuk H, Naldoken S. Brain perfusion abnormalities in chronic obstructive pulmonary disease: comparison with cognitive impairment. Ann Nucl Med. 2006; 20:99–106.19. Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004; 44:109–120.20. Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990; 13:171–182.21. Yendiki A, Koldewyn K, Kakunoori S, Kanwisher N, Fischl B. Spurious group differences due to head motion in a diffusion MRI study. Neuroimage. 2014; 88:79–90.22. Greve DN, Fischl B. Accurate and robust brain image alignment using boundary-based registration. Neuroimage. 2009; 48:63–72.23. Marino-Neto J, Sabbatini RM. A stereotaxic atlas for the telencephalon of the Siamese fighting fish (Betta splendens). Braz J Med Biol Res. 1988; 21:971–986.24. Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002; 17:825–841.25. Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999; 9:179–194.26. Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999; 9:195–207.27. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002; 33:341–355.28. Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000; 97:11050–11055.29. Fischl B, Salat DH, van der Kouwe AJ, Makris N, Ségonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004; 23:Suppl 1. S69–S84.30. Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage. 2007; 34:144–155.31. Tae WS, Kim SS, Lee KU, Nam EC, Kim KW. Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology. 2008; 50:569–581.32. Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005; 15:854–869.33. Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007; 4:316–329.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Association of White Matter Tracts with Alexithymia among Individuals with Major Depressive Disorder
- Association Between the C4 Binding Protein Level and White Matter Integrity in Major Depressive Disorder
- Association Between White Matter Tract Integrity and Frontal-Executive Function in Non-Geriatric Adult Patients With Major Depressive Disorder
- Diffusion Tensor Imaging Findings of White Matter Changes in First Episode Schizophrenia: A Systematic Review
- Factors Affecting the Perception, Knowledge, and Preventive Behaviors of Chronic Pulmonary Disease Patients on Particulate Matter