Korean J Crit Care Med.
2017 Nov;32(4):307-322. 10.4266/kjccm.2017.00535.
Patient-Ventilator Dyssynchrony
- Affiliations
-
- 1Intensive Care Unit, University Hospital of Heraklion, Heraklion, Greece. akoumianakievangelia@gmail.com
- 2Medical School, University of Crete, Heraklion, Greece.
- KMID: 2405117
- DOI: http://doi.org/10.4266/kjccm.2017.00535
Abstract
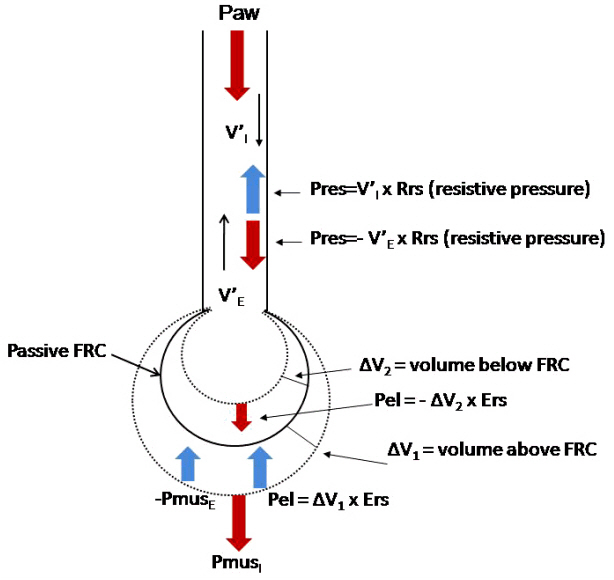
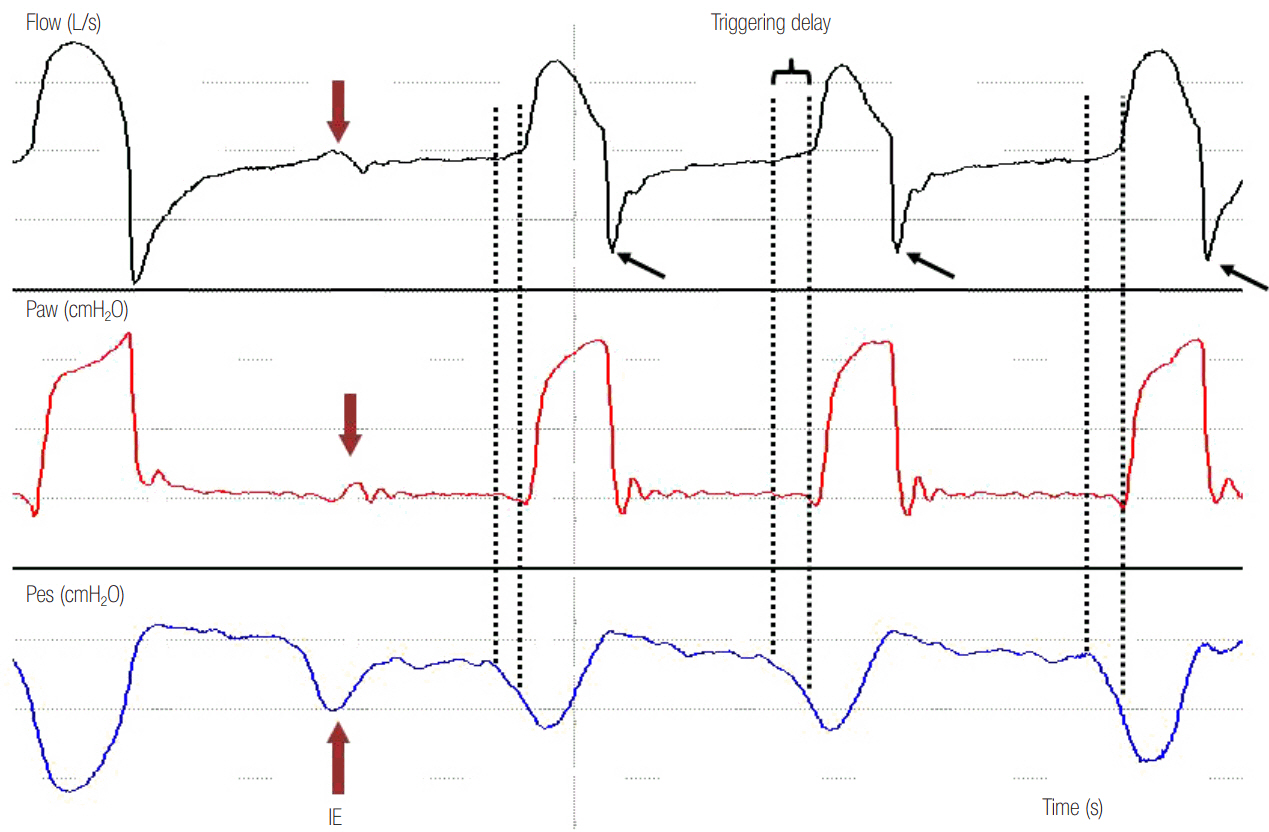
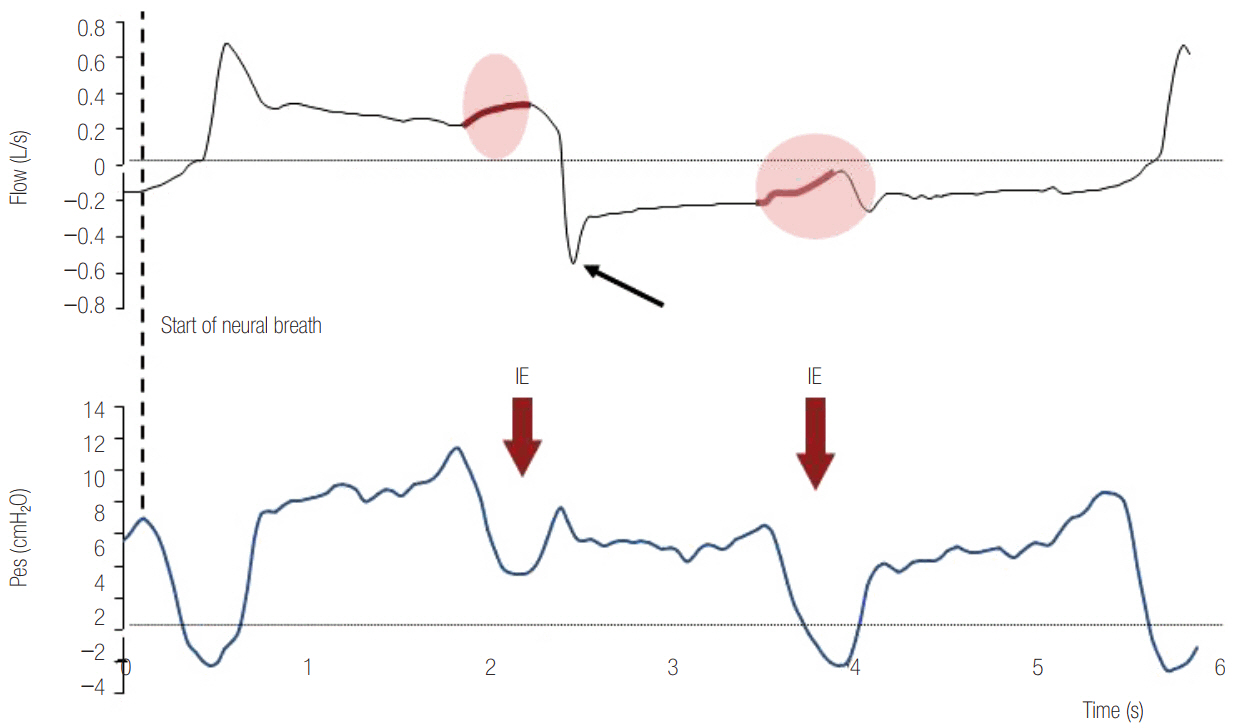
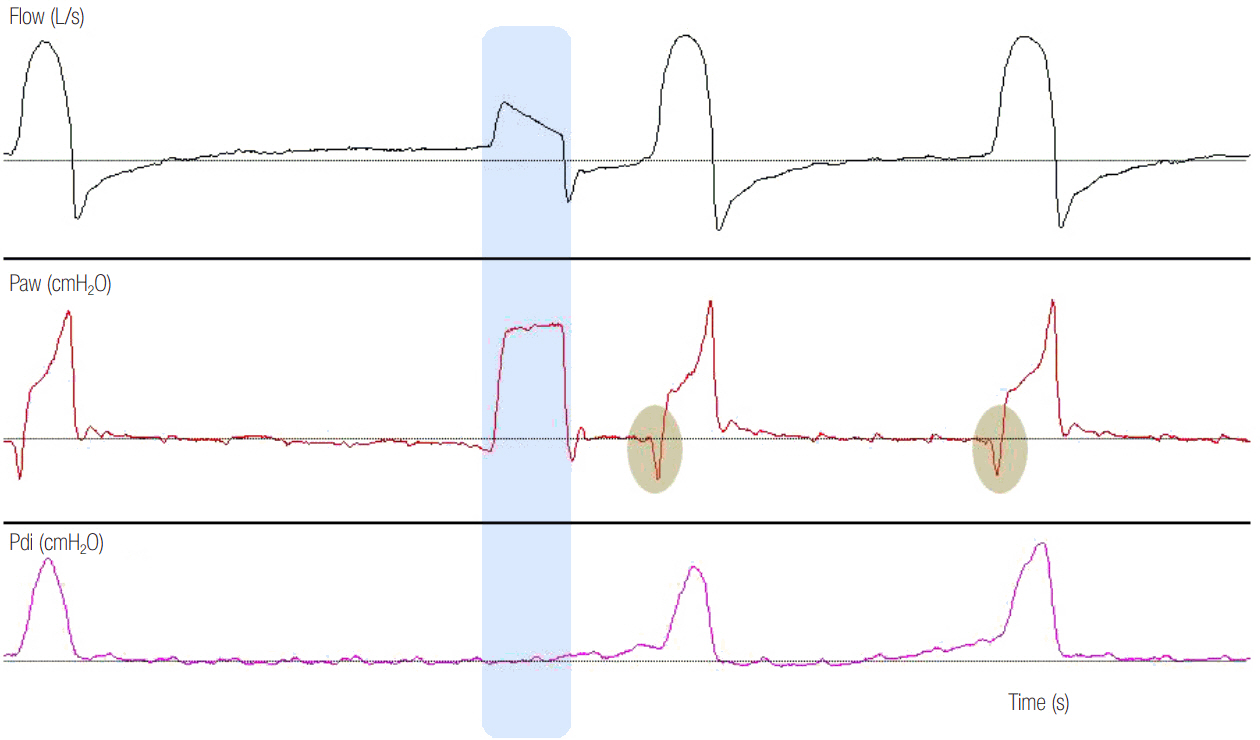
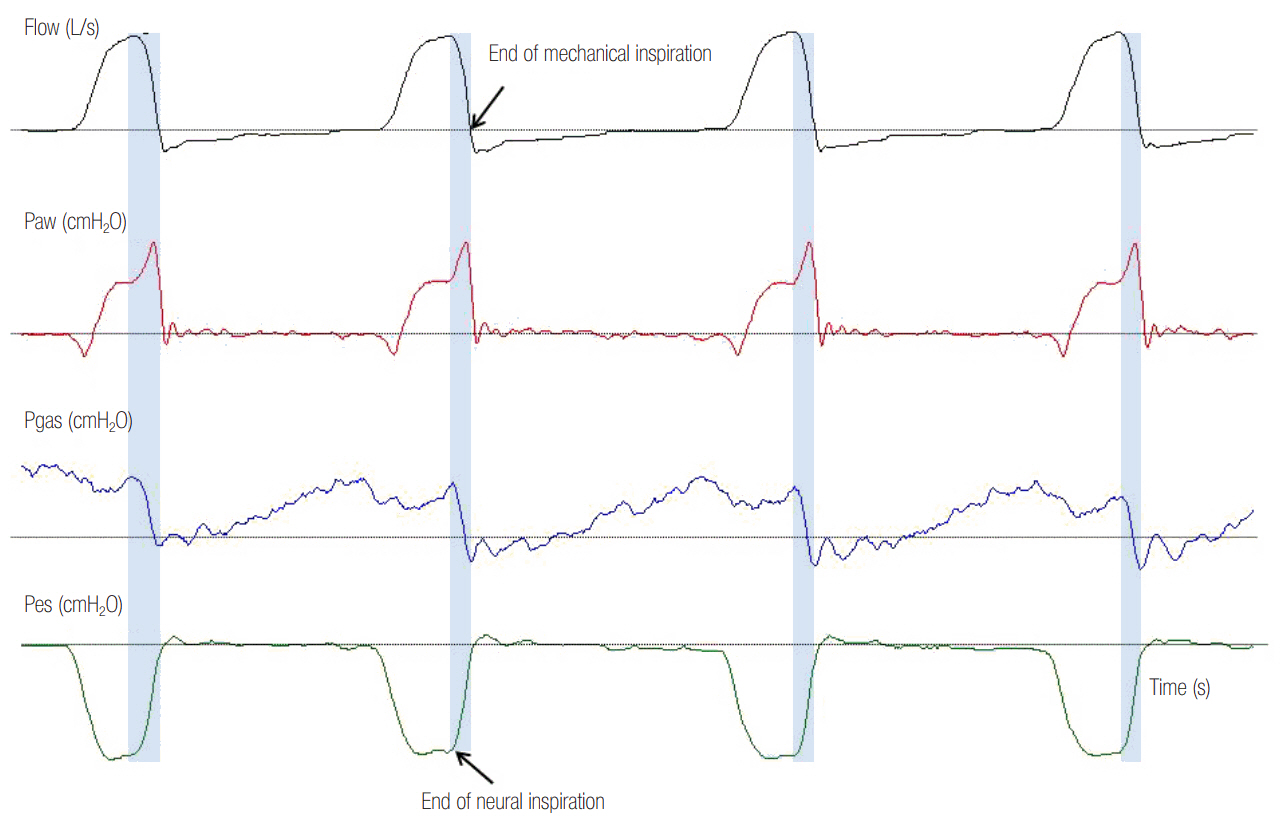
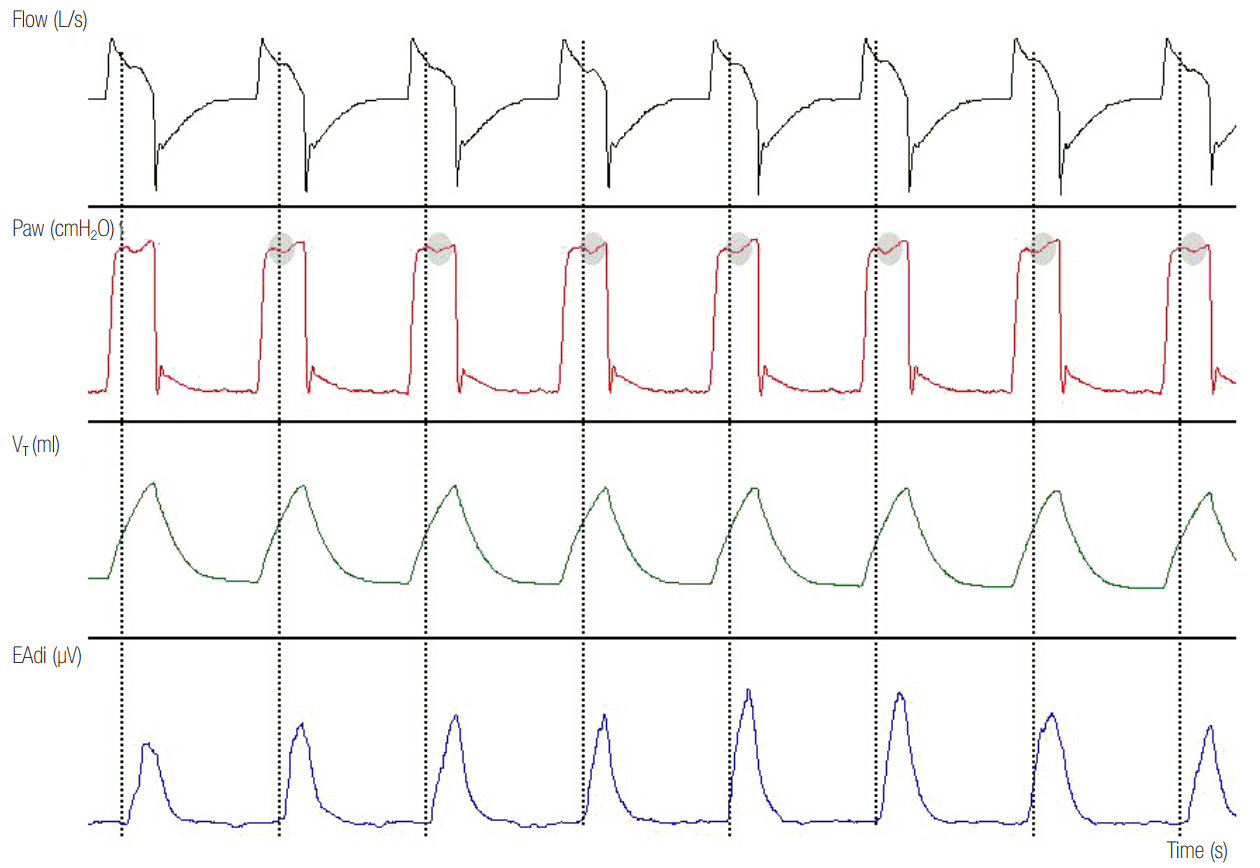
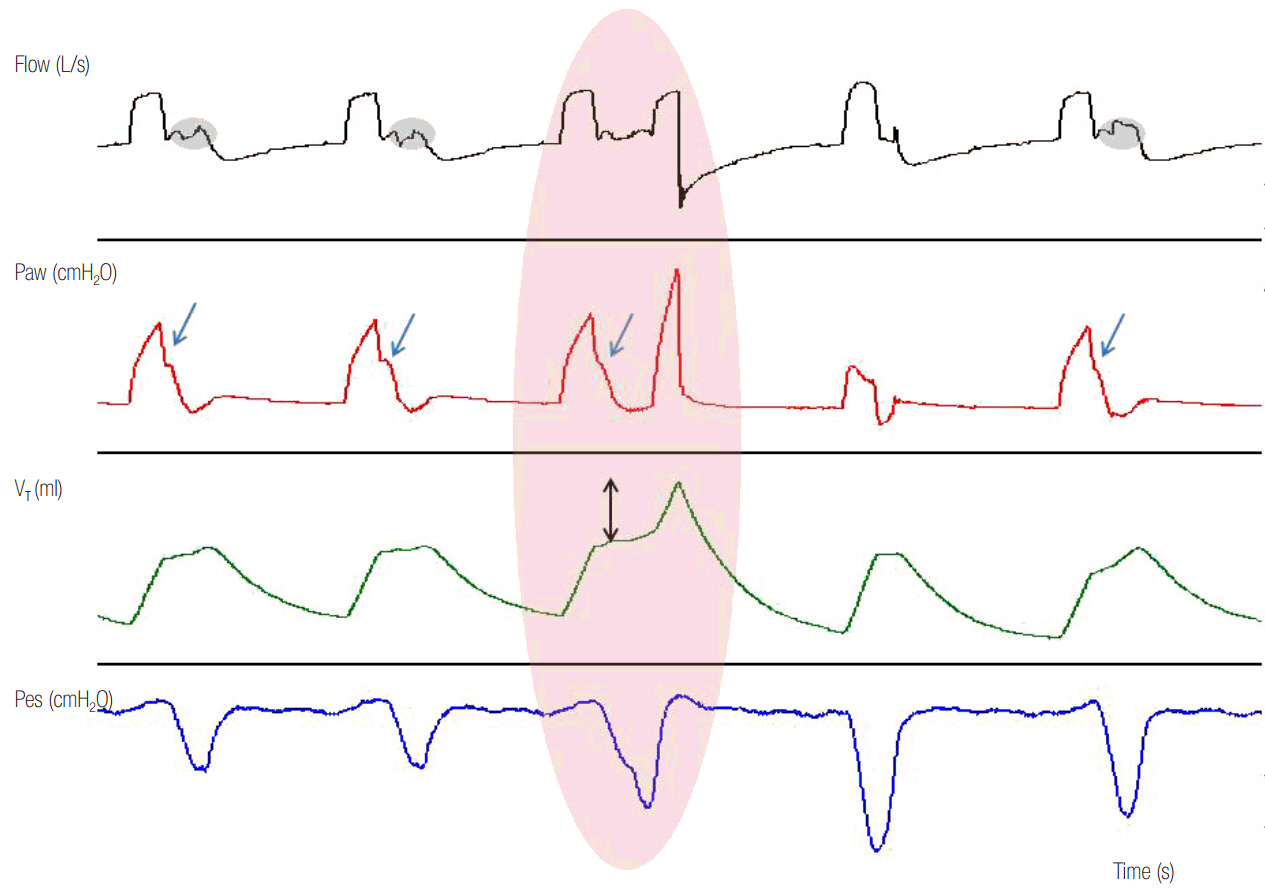
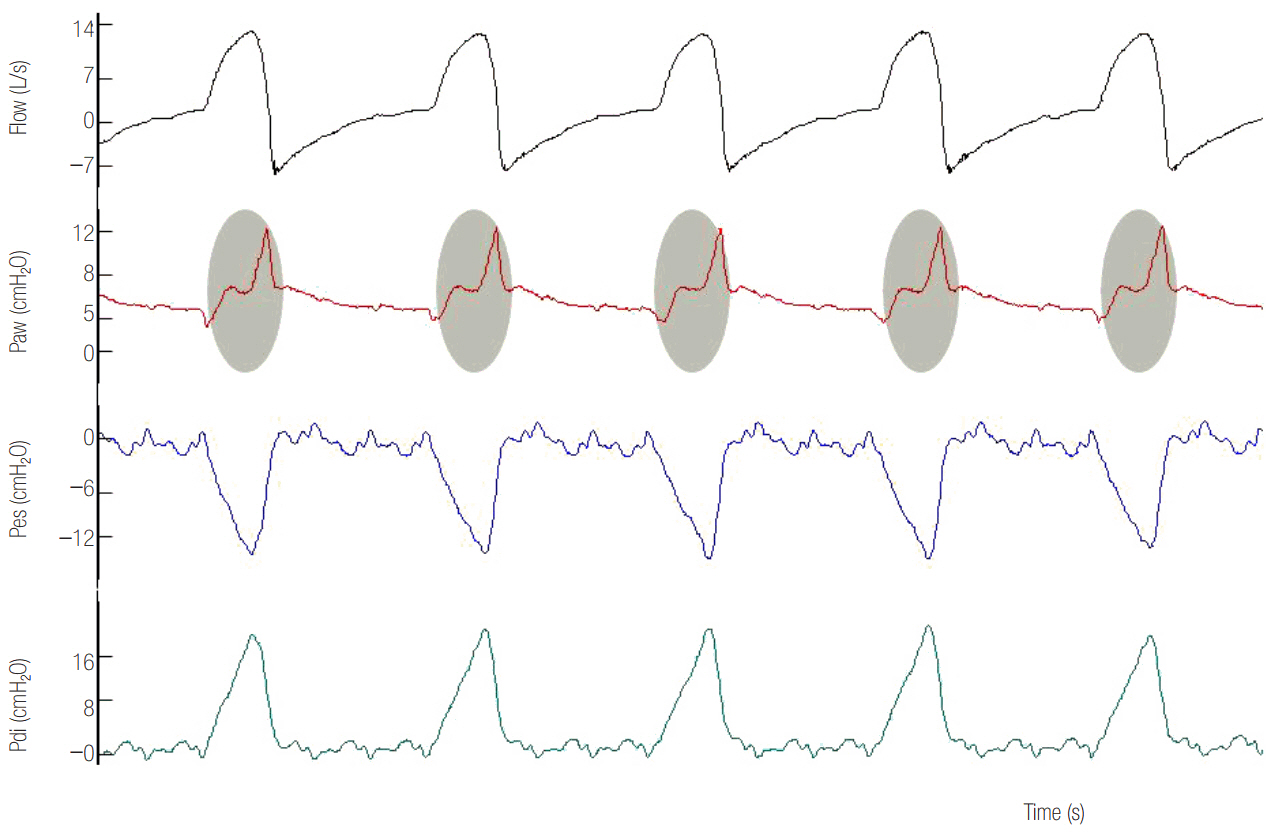
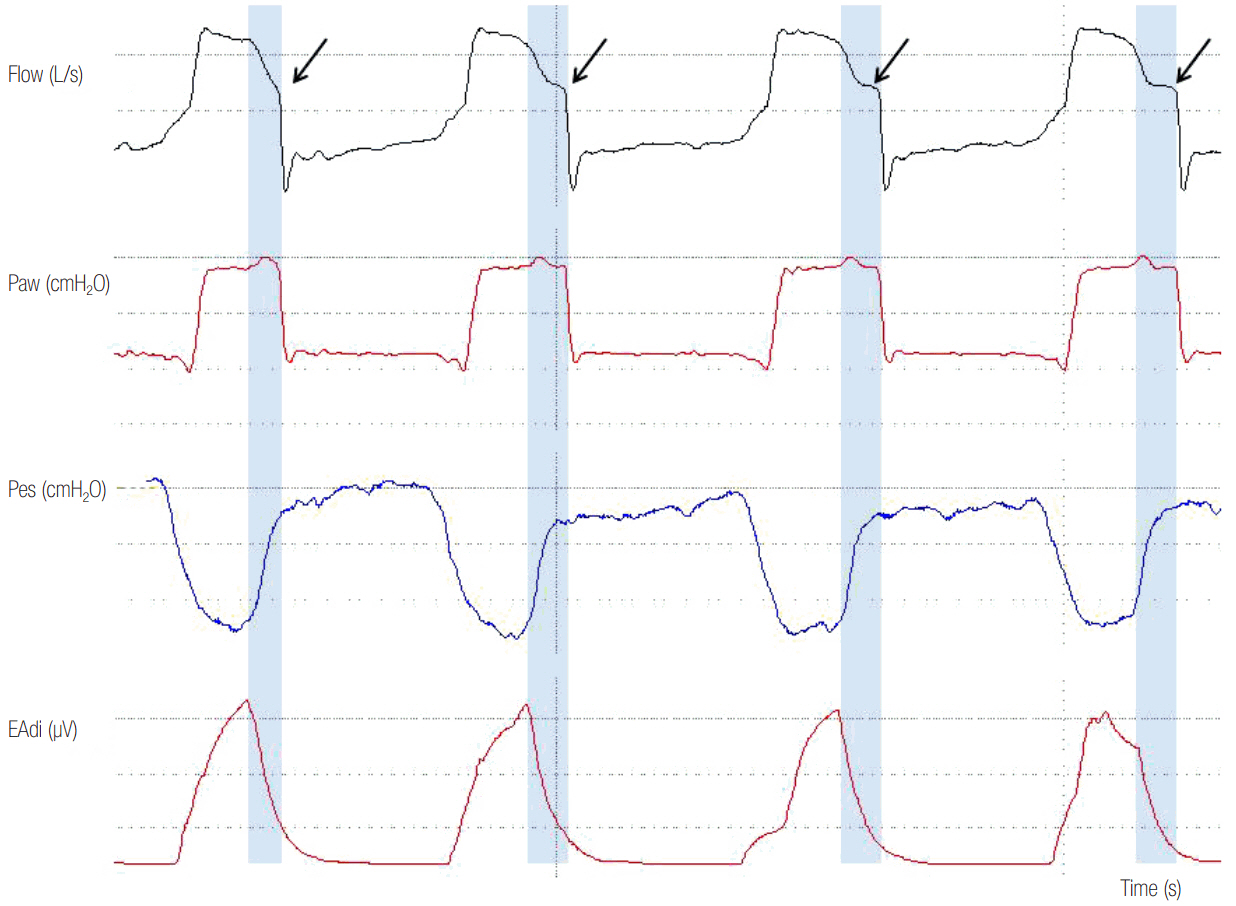
- In mechanically ventilated patients, assisted mechanical ventilation (MV) is employed early, following the acute phase of critical illness, in order to eliminate the detrimental effects of controlled MV, most notably the development of ventilator-induced diaphragmatic dysfunction. Nevertheless, the benefits of assisted MV are often counteracted by the development of patient-ventilator dyssynchrony. Patient-ventilator dyssynchrony occurs when either the initiation and/or termination of mechanical breath is not in time agreement with the initiation and termination of neural inspiration, respectively, or if the magnitude of mechanical assist does not respond to the patient's respiratory demand. As patient-ventilator dyssynchrony has been associated with several adverse effects and can adversely influence patient outcome, every effort should be made to recognize and correct this occurrence at bedside. To detect patient-ventilator dyssynchronies, the physician should assess patient comfort and carefully inspect the pressure- and flowtime waveforms, available on the ventilator screen of all modern ventilators. Modern ventilators offer several modifiable settings to improve patient-ventilator interaction. New proportional modes of ventilation are also very helpful in improving patient-ventilator interaction.
Figure
Reference
-
References
1. Petrof BJ, Jaber S, Matecki S. Ventilator-induced diaphragmatic dysfunction. Curr Opin Crit Care. 2010; 16:19–25.
Article2. Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008; 358:1327–35.
Article3. Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000; 342:1471–7.
Article4. Schweickert WD, Gehlbach BK, Pohlman AS, Hall JB, Kress JP. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004; 32:1272–6.
Article5. Garnacho-Montero J, Madrazo-Osuna J, Garcia- Garmendia JL, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar A, et al. Critical illness polyneuropathy: risk factors and clinical consequences. A cohort study in septic patients. Intensive Care Med. 2001; 27:1288–96.
Article6. Putensen C, Zech S, Wrigge H, Zinserling J, Stuber F, Von Spiegel T, et al. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001; 164:43–9.
Article7. de Wit M, Miller KB, Green DA, Ostman HE, Gennings C, Epstein SK. Ineffective triggering predicts increased duration of mechanical ventilation. Crit Care Med. 2009; 37:2740–5.
Article8. Thille AW, Rodriguez P, Cabello B, Lellouche F, Brochard L. Patient-ventilator asynchrony during assisted mechanical ventilation. Intensive Care Med. 2006; 32:1515–22.
Article9. Blanch L, Villagra A, Sales B, Montanya J, Lucangelo U, Lujan M, et al. Asynchronies during mechanical ventilation are associated with mortality. Intensive Care Med. 2015; 41:633–41.
Article10. Bosma K, Ferreyra G, Ambrogio C, Pasero D, Mirabella L, Braghiroli A, et al. Patient-ventilator interaction and sleep in mechanically ventilated patients: pressure support versus proportional assist ventilation. Crit Care Med. 2007; 35:1048–54.
Article11. Georgopoulos D, Prinianakis G, Kondili E. Bedside waveforms interpretation as a tool to identify patient-ventilator asynchronies. Intensive Care Med. 2006; 32:34–47.
Article12. Sassoon CS, Gruer SE. Characteristics of the ventilator pressure- and flow-trigger variables. Intensive Care Med. 1995; 21:159–68.13. Aslanian P, El Atrous S, Isabey D, Valente E, Corsi D, Harf A, et al. Effects of flow triggering on breathing effort during partial ventilatory support. Am J Respir Crit Care Med. 1998; 157:135–43.
Article14. Goulet R, Hess D, Kacmarek RM. Pressure vs flow triggering during pressure support ventilation. Chest. 1997; 111:1649–53.
Article15. Prinianakis G, Kondili E, Georgopoulos D. Effects of the flow waveform method of triggering and cycling on patient-ventilator interaction during pressure support. Intensive Care Med. 2003; 29:1950–9.
Article16. Racca F, Squadrone V, Ranieri VM. Patient-ventilator interaction during the triggering phase. Respir Care Clin N Am. 2005; 11:225–45.
Article17. Slutsky AS. Mechanical ventilation: American College of Chest Physicians’ Consensus Conference. Chest. 1993; 104:1833–59.18. Prinianakis G, Kondili E, Georgopoulos D. Patient-ventilator interaction: an overview. Respir Care Clin N Am. 2005; 11:201–24.
Article19. Tobin MJ, Jubran A, Laghi F. Patient-ventilator interaction. Am J Respir Crit Care Med. 2001; 163:1059–63.
Article20. Sharshar T, Desmarais G, Louis B, Macadou G, Porcher R, Harf A, et al. Transdiaphragmatic pressure control of airway pressure support in healthy subjects. Am J Respir Crit Care Med. 2003; 168:760–9.
Article21. Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, et al. Neural control of mechanical ventilation in respiratory failure. Nat Med. 1999; 5:1433–6.
Article22. Kondili E, Xirouchaki N, Georgopoulos D. Modulation and treatment of patient-ventilator dyssynchrony. Curr Opin Crit Care. 2007; 13:84–9.
Article23. Georgopoulos DB, Anastasaki M, Katsanoulas K. Effects of mechanical ventilation on control of breathing. Monaldi Arch Chest Dis. 1997; 52:253–62.24. Brander L, Leong-Poi H, Beck J, Brunet F, Hutchison SJ, Slutsky AS, et al. Titration and implementation of neurally adjusted ventilatory assist in critically ill patients. Chest. 2009; 135:695–703.
Article25. Bonmarchand G, Chevron V, Chopin C, Jusserand D, Girault C, Moritz F, et al. Increased initial flow rate reduces inspiratory work of breathing during pressure support ventilation in patients with exacerbation of chronic obstructive pulmonary disease. Intensive Care Med. 1996; 22:1147–54.
Article26. Bonmarchand G, Chevron V, Ménard JF, Girault C, Moritz-Berthelot F, Pasquis P, et al. Effects of pressure ramp slope values on the work of breathing during pressure support ventilation in restrictive patients. Crit Care Med. 1999; 27:715–22.
Article27. Chiumello D, Pelosi P, Taccone P, Slutsky A, Gattinoni L. Effect of different inspiratory rise time and cycling off criteria during pressure support ventilation in patients recovering from acute lung injury. Crit Care Med. 2003; 31:2604–10.
Article28. Chiumello D, Pelosi P, Calvi E, Bigatello LM, Gattinoni L. Different modes of assisted ventilation in patients with acute respiratory failure. Eur Respir J. 2002; 20:925–33.
Article29. Thille AW, Cabello B, Galia F, Lyazidi A, Brochard L. Reduction of patient-ventilator asynchrony by reducing tidal volume during pressure-support ventilation. Intensive Care Med. 2008; 34:1477–86.
Article30. Leung P, Jubran A, Tobin MJ. Comparison of assisted ventilator modes on triggering, patient effort, and dyspnea. Am J Respir Crit Care Med. 1997; 155:1940–8.
Article31. Younes M, Kun J, Webster K, Roberts D. Response of ventilator-dependent patients to delayed opening of exhalation valve. Am J Respir Crit Care Med. 2002; 166:21–30.
Article32. Vaporidi K, Babalis D, Chytas A, Lilitsis E, Kondili E, Amargianitakis V, et al. Clusters of ineffective efforts during mechanical ventilation: impact on outcome. Intensive Care Med. 2017; 43:184–91.
Article33. Vitacca M, Bianchi L, Zanotti E, Vianello A, Barbano L, Porta R, et al. Assessment of physiologic variables and subjective comfort under different levels of pressure support ventilation. Chest. 2004; 126:851–9.
Article34. Nava S, Bruschi C, Rubini F, Palo A, Iotti G, Braschi A. Respiratory response and inspiratory effort during pressure support ventilation in COPD patients. Intensive Care Med. 1995; 21:871–9.
Article35. Rossi A, Polese G, Brandi G, Conti G. Intrinsic positive end-expiratory pressure (PEEPi). Intensive Care Med. 1995; 21:522–36.
Article36. Fabry B, Guttmann J, Eberhard L, Bauer T, Haberthür C, Wolff G. An analysis of desynchronization between the spontaneously breathing patient and ventilator during inspiratory pressure support. Chest. 1995; 107:1387–94.
Article37. Imanaka H, Nishimura M, Takeuchi M, Kimball WR, Yahagi N, Kumon K. Autotriggering caused by cardiogenic oscillation during flow-triggered mechanical ventilation. Crit Care Med. 2000; 28:402–7.
Article38. Hill LL, Pearl RG. Flow triggering, pressure triggering, and autotriggering during mechanical ventilation. Crit Care Med. 2000; 28:579–81.
Article39. Carteaux G, Lyazidi A, Cordoba-Izquierdo A, Vignaux L, Jolliet P, Thille AW, et al. Patient-ventilator asynchrony during noninvasive ventilation: a bench and clinical study. Chest. 2012; 142:367–76.40. Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med. 2004; 170:626–32.
Article41. Du HL, Yamada Y. Expiratory asynchrony. Respir Care Clin N Am. 2005; 11:265–80.
Article42. Prinianakis G, Plataki M, Kondili E, Klimathianaki M, Vaporidi K, Georgopoulos D. Effects of relaxation of inspiratory muscles on ventilator pressure during pressure support. Intensive Care Med. 2008; 34:70–4.43. Kondili E, Prinianakis G, Georgopoulos D. Patient-ventilator interaction. Br J Anaesth. 2003; 91:106–19.
Article44. Yamada Y, Du HL. Analysis of the mechanisms of expiratory asynchrony in pressure support ventilation: a mathematical approach. J Appl Physiol (1985). 2000; 88:2143–50.45. Akoumianaki E, Lyazidi A, Rey N, Matamis D, Perez- Martinez N, Giraud R, et al. Mechanical ventilation-induced reverse-triggered breaths: a frequently unrecognized form of neuromechanical coupling. Chest. 2013; 143:927–38.46. Simon PM, Habel AM, Daubenspeck JA, Leiter JC. Vagal feedback in the entrainment of respiration to mechanical ventilation in sleeping humans. J Appl Physiol (1985). 2000; 89:760–9.
Article47. Simon PM, Zurob AS, Wies WM, Leiter JC, Hubmayr RD. Entrainment of respiration in humans by periodic lung inflations: effect of state and CO(2). Am J Respir Crit Care Med. 1999; 160:950–60.48. Meza S, Mendez M, Ostrowski M, Younes M. Susceptibility to periodic breathing with assisted ventilation during sleep in normal subjects. J Appl Physiol (1985). 1998; 85:1929–40.49. Tobin MJ. Monitoring of pressure, flow, and volume during mechanical ventilation. Respir Care. 1992; 37:1081–96.50. Jubran A, Van de Graaff WB, Tobin MJ. Variability of patient-ventilator interaction with pressure support ventilation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995; 152:129–36.
Article51. Terzi N, Pelieu I, Guittet L, Ramakers M, Seguin A, Daubin C, et al. Neurally adjusted ventilatory assist in patients recovering spontaneous breathing after acute respiratory distress syndrome: physiological evaluation. Crit Care Med. 2010; 38:1830–7.
Article52. Spahija J, de Marchie M, Albert M, Bellemare P, Delisle S, Beck J, et al. Patient-ventilator interaction during pressure support ventilation and neurally adjusted ventilatory assist. Crit Care Med. 2010; 38:518–26.
Article53. Xirouchaki N, Kondili E, Vaporidi K, Xirouchakis G, Klimathianaki M, Gavriilidis G, et al. Proportional assist ventilation with load-adjustable gain factors in critically ill patients: comparison with pressure support. Intensive Care Med. 2008; 34:2026–34.
Article54. Piquilloud L, Vignaux L, Bialais E, Roeseler J, Sottiaux T, Laterre PF, et al. Neurally adjusted ventilatory assist improves patient-ventilator interaction. Intensive Care Med. 2011; 37:263–71.
Article55. Kondili E, Prinianakis G, Alexopoulou C, Vakouti E, Klimathianaki M, Georgopoulos D. Respiratory load compensation during mechanical ventilation: proportional assist ventilation with load-adjustable gain factors versus pressure support. Intensive Care Med. 2006; 32:692–9.56. Lecomte F, Brander L, Jalde F, Beck J, Qui H, Elie C, et al. Physiological response to increasing levels of neurally adjusted ventilatory assist (NAVA). Respir Physiol Neurobiol. 2009; 166:117–24.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of Left Bundle Branch Block on Left Atrial Dyssynchrony and Its Relationship to Left Ventricular Diastolic Function in Patients with Heart Failure and Dilated Cardiomyopathy
- Left Ventricular Dyssynchrony in Patients Showing Diastolic Dysfunction without Overt Symptoms of Heart Failure
- Ventricular dyssynchrony in patients with permanent pacemaker
- Clinical Implication of Mechanical Dyssynchrony in Heart Failure
- Does the " Curare Cleft " on the Capnogram always mean that the Patient is in Need of Relaxant ?