Ann Lab Med.
2018 May;38(3):235-241. 10.3343/alm.2018.38.3.235.
Same-Day Identification and Antimicrobial Susceptibility Testing of Bacteria in Positive Blood Culture Broths Using Short-Term Incubation on Solid Medium with the MicroFlex LT, Vitek-MS, and Vitek2 Systems
- Affiliations
-
- 1Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea. deyong@yuhs.ac
- 2Department of Laboratory Medicine, National Cancer Center, Goyang, Korea. skhong@ncc.re.kr
- KMID: 2403457
- DOI: http://doi.org/10.3343/alm.2018.38.3.235
Abstract
- BACKGROUND
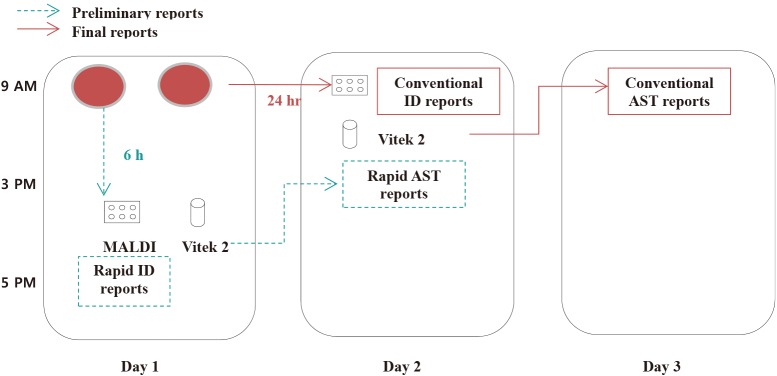
Early and appropriate antibiotic treatment improves the clinical outcome of patients with septicemia; therefore, reducing the turn-around time for identification (ID) and antimicrobial susceptibility test (AST) results is essential. We established a method for rapid ID and AST using short-term incubation of positive blood culture broth samples on solid media, and evaluated its performance relative to that of the conventional method using two rapid ID systems and a rapid AST method.
METHODS
A total of 254 mono-microbial samples were included. Positive blood culture samples were incubated on blood agar plates for six hours and identified by the MicroFlex LT (Bruker Daltonics) and Vitek-MS (bioMeriéux) systems, followed by AST using the Vitek2 System (bioMeriéux).
RESULTS
The correct species-level ID rates were 82.3% (209/254) and 78.3% (199/254) for the MicroFlex LT and Vitek-MS platforms, respectively. For the 1,174 microorganism/antimicrobial agent combinations tested, the rapid AST method showed total concordance of 97.8% (1,148/1,174) with the conventional method, with a very major error rate of 0.5%, major error rate of 0.7%, and minor error rate of 1.0%.
CONCLUSIONS
Routine implementation of this short-term incubation method could provide ID results on the day of blood culture-positivity detection and one day earlier than the conventional AST method. This simple method will be very useful for rapid ID and AST of bacteria from positive blood culture bottles in routine clinical practice.
Keyword
Figure
Reference
-
1. Paul M, Shani V, Muchtar E, Kariv G, Robenshtok E, Leibovici L. Systematic review and meta-analysis of the efficacy of appropriate empiric antibiotic therapy for sepsis. Antimicrob Agents Chemother. 2010; 54:4851–4863. PMID: 20733044.2. Seng P, Rolain JM, Fournier PE, La Scola B, Drancourt M, Raoult D. MALDI-TOF-mass spectrometry applications in clinical microbiology. Future Microbiol. 2010; 5:1733–1754. PMID: 21133692.3. Machen A, Drake T, Wang YF. Same day identification and full panel antimicrobial susceptibility testing of bacteria from positive blood culture bottles made possible by a combined lysis-filtration method with MALDI-TOF VITEK mass spectrometry and the VITEK2 system. PLoS One. 2014; 9:e87870. PMID: 24551067.4. Prod'hom G, Bizzini A, Durussel C, Bille J, Greub G. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for direct bacterial identification from positive blood culture pellets. J Clin Microbiol. 2010; 48:1481–1483. PMID: 20164269.5. Monteiro J, Inoue FM, Lobo AP, Sugawara EK, Boaretti FM, Tufik S. Fast and reliable bacterial identification direct from positive blood culture using a new TFA sample preparation protocol and the Vitek® MS system. J Microbiol Methods. 2015; 109:157–159. PMID: 25541363.6. Jakovljev A, Bergh K. Development of a rapid and simplified protocol for direct bacterial identification from positive blood cultures by using matrix-assisted laser desorption ionization time-of- flight mass spectrometry. BMC Microbiol. 2015; 15:258. PMID: 26546000.7. Jo SJ, Park KG, Han K, Park DJ, Park YJ. Direct identification and antimicrobial susceptibility testing of bacteria from positive blood culture bottles by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and the Vitek2 system. Ann Lab Med. 2016; 36:117–123. PMID: 26709258.8. Chen JH, Ho PL, Kwan GS, She KK, Siu GK, Cheng VC, et al. Direct bacterial identification in positive blood cultures by use of two commercial matrix-assisted laser desorption ionization-time of flight mass spectrometry systems. J Clin Microbiol. 2013; 51:1733–1739. PMID: 23515548.9. Buchan BW, Riebe KM, Ledeboer NA. Comparison of the MALDI Biotyper system using Sepsityper sample processing to routine microbiological methods for identification of bacteria from positive blood culture bottles. J Clin Microbiol. 2012; 50:346–352. PMID: 22162549.10. CLSI. CLSI supplement M100-S24. Performance standards for antimicrobial susceptibility testing. 24th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2014.11. Diekema DJ, Beekmann SE, Chapin KC, Morel KA, Munson E, Doern GV. Epidemiology and outcome of nosocomial and community-onset bloodstream infection. J Clin Microbiol. 2003; 41:3655–3660. PMID: 12904371.12. Huang AM, Newton D, Kunapuli A, Gandhi TN, Washer LL, Isip J, et al. Impact of rapid organism identification via matrix-assisted laser desorption/ ionization time-of-flight combined with antimicrobial stewardship team intervention in adult patients with bacteremia and candidemia. Clin Infect Dis. 2013; 57:1237–1245. PMID: 23899684.13. Vlek AL, Bonten MJ, Boel CH. Direct matrix-assisted laser desorption ionization time-of-flight mass spectrometry improves appropriateness of antibiotic treatment of bacteremia. PLoS One. 2012; 7:e32589. PMID: 22438880.14. Kohlmann R, Hoffmann A, Geis G, Gatermann S. MALDI-TOF mass spectrometry following short incubation on a solid medium is a valuable tool for rapid pathogen identification from positive blood cultures. Int J Med Microbiol. 2015; 305:469–479. PMID: 25953498.15. Zabbe JB, Zanardo L, Mégraud F, Bessède E. MALDI-TOF mass spectrometry for early identification of bacteria grown in blood culture bottles. J Microbiol Methods. 2015; 115:45–46. PMID: 25940929.16. Hong SK, Chang BK, Song SH, Kim EC. Use of MALDI-TOF MS technique for rapid identification of bacteria from positive blood cultures. Indian J Med Microbiol. 2014; 32:419–422. PMID: 25297028.17. Bhatti MM, Boonlayangoor S, Beavis KG, Tesic V. Rapid identification of positive blood cultures by matrix-assisted laser desorption ionizationtime of flight mass spectrometry using prewarmed agar plates. J Clin Microbiol. 2014; 52:4334–4338. PMID: 25232166.18. Fan WT, Qin TT, Bi RR, Kang HQ, Ma P, Gu B. Performance of the matrix-assisted laser desorption ionization time-of-flight mass spectrometry system for rapid identification of streptococci: a review. Eur J Clin Microbiol Infect Dis. 2017; 36:1005–1012. PMID: 28116553.19. Center for Devices and Radiological Health, Food and Drug Administration. Class II Special Controls Guidance Document: Antimicrobial Susceptibility Test (AST) Systems. Updated on Aug 2009. https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm388961.pdf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Direct Identification and Antimicrobial Susceptibility Testing of Bacteria From Positive Blood Culture Bottles by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry and the Vitek 2 System
- Comparison of the New VITEK MS PRIME System with the Matrix-Assisted Laser Desorption Ionization Biotyper Microflex LT for the Identification of Microorganisms
- A Case of Chryseobacterium hominis Isolated from Human Blood Drawn Through Peripherally Inserted Central Catheter
- Changes in Antimicrobial Susceptibility Pattern of Blood Isolates at a University Hospital in the Kyungnam area during 2005–2014
- Performance of Microflex LT Biotyper and VITEK MS for Routine Identification of Yeasts