Cancer Res Treat.
2015 Oct;47(4):823-833. 10.4143/crt.2014.074.
APE1/Ref-1 as a Serological Biomarker for the Detection of Bladder Cancer
- Affiliations
-
- 1Department of Urology, Chungnam National University Hospital, Daejeon, Korea. uro17@cnuh.co.kr
- 2Infection Signaling Network Research Center, Research Institute of Medical Sciences, Department of Physiology, Chungnam National University School of Medicine, Daejeon, Korea. bhjeon@cnu.ac.kr
- 3Division of Cardiovascular Medicine, Department of Internal Medicine, University of Iowa Carver College of Medicine, Iowa City, IA, USA.
- 4Department of Pathology, Chungnam National University School of Medicine, Daejeon, Korea.
- KMID: 2403402
- DOI: http://doi.org/10.4143/crt.2014.074
Abstract
- PURPOSE
Apurinic/apyrimidinic endonuclease 1/redox factor-1 (APE1/Ref-1) is a multifunctional protein that shows elevated expression in a number of cancers. We attempted to determine whether serum APE1/Ref-1 is elevated in patients with bladder cancer.
MATERIALS AND METHODS
Serum APE1/Ref-1 levels were determined using enzyme-linked immunosorbent assay in serum from patients with bladder cancer who had not received chemotherapy or radiotherapy (n=51) and non-tumor controls (n=55). The area under the receiver operating characteristic area under the curve was applied to determine the correlation between clinical factors and the serum levels of APE1/Ref-1.
RESULTS
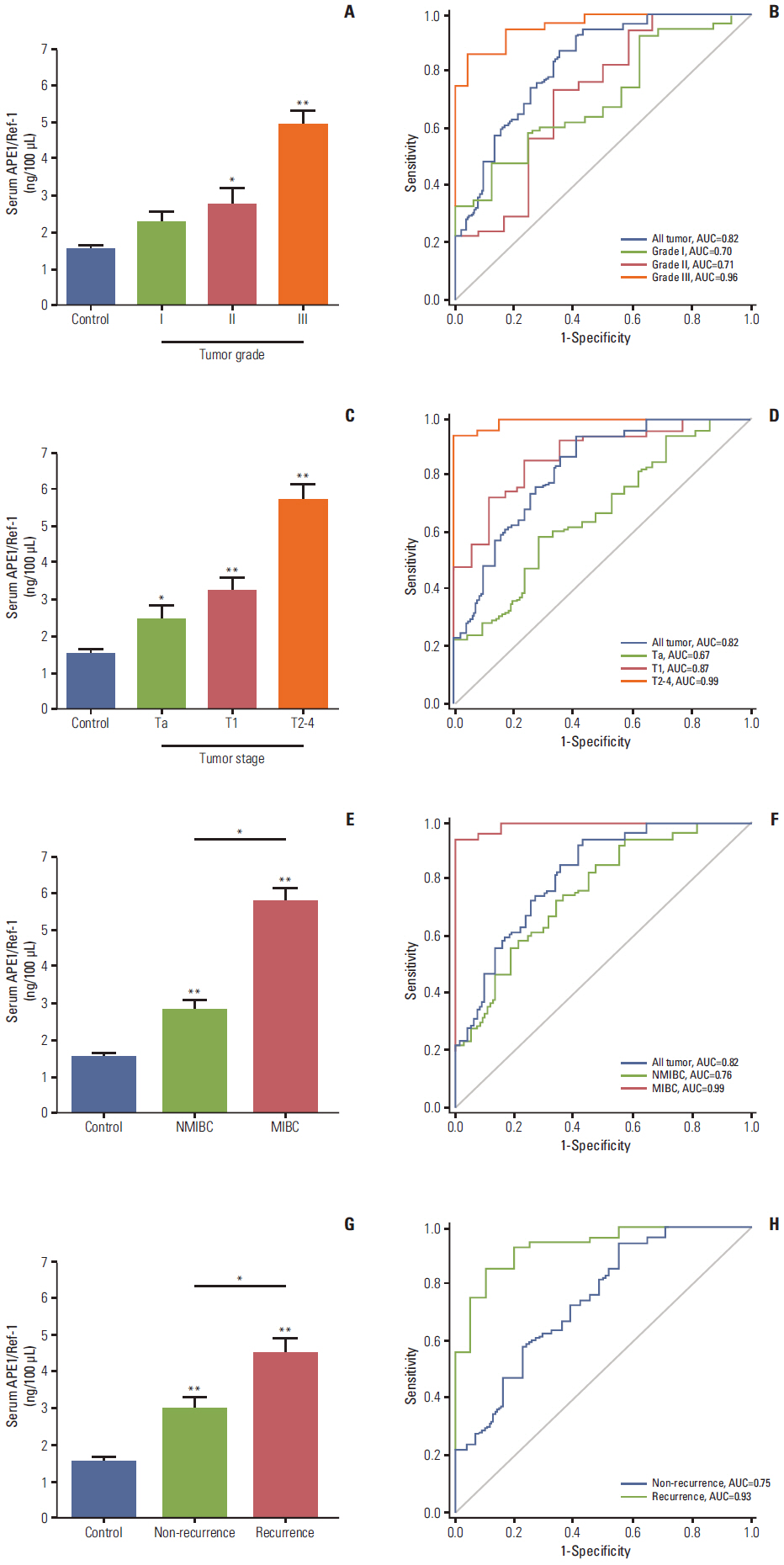
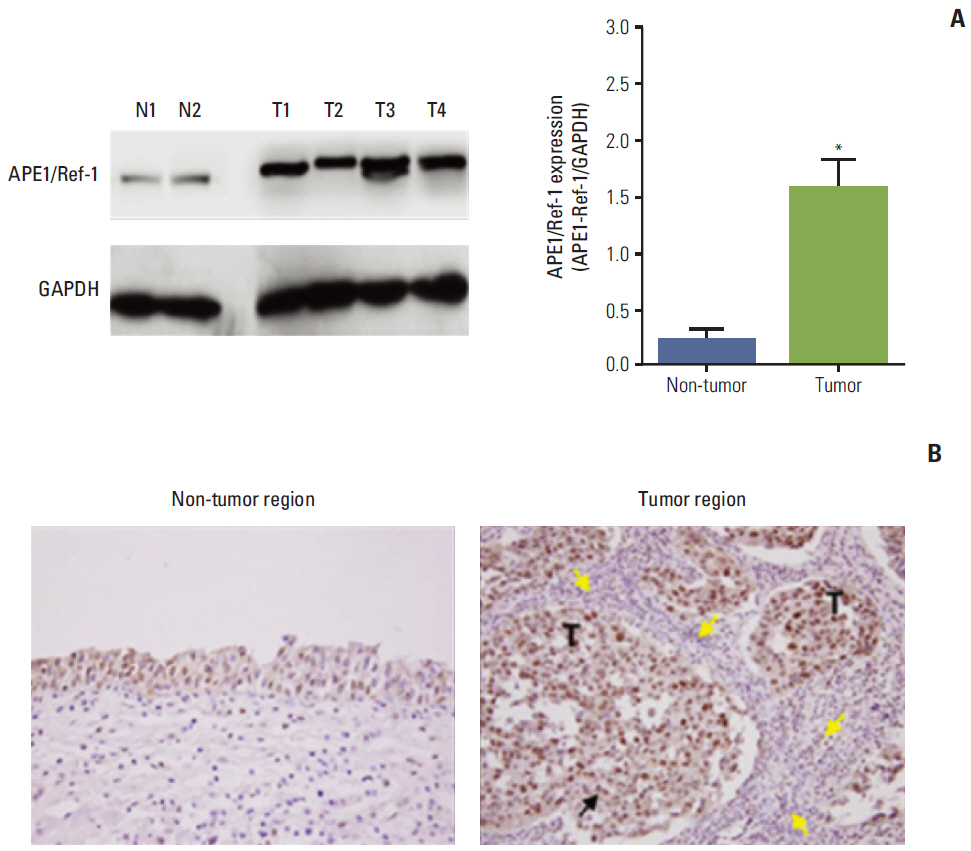
Serum levels of APE1/Ref-1 in bladder cancer patients were significantly elevated compared to those of the control group (3.548+/-0.333 ng/100 muL [n=51] for bladder cancer vs. 1.547+/-0.319 ng/100 muL [n=55] for the control group), with a sensitivity and specificity of 93% and 59%, respectively. Serum APE1/Ref-1 levels are associated with tumor stage, grade, muscle invasion, and recurrence.
CONCLUSION
Serum APE1/Ref-1 might be useful as a potential serologic biomarker for bladder cancer.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Dynamic Regulation of APE1/Ref-1 as a Therapeutic Target Protein
Sunga Choi, Hee Kyoung Joo, Byeong Hwa Jeon
Chonnam Med J. 2016;52(2):75-80. doi: 10.4068/cmj.2016.52.2.75.
Reference
-
References
1. Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012; 62:220–41.
Article2. Jung KW, Park S, Kong HJ, Won YJ, Lee JY, Park EC, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2008. Cancer Res Treat. 2011; 43:1–11.
Article3. Lamm DL, Blumenstein BA, Crissman JD, Montie JE, Gottesman JE, Lowe BA, et al. Maintenance bacillus Calmette-Guerin immunotherapy for recurrent TA, T1 and carcinoma in situ transitional cell carcinoma of the bladder: a randomized Southwest Oncology Group Study. J Urol. 2000; 163:1124–9.
Article4. Vrooman OP, Witjes JA. Molecular markers for detection, surveillance and prognostication of bladder cancer. Int J Urol. 2009; 16:234–43.
Article5. Raab SS, Grzybicki DM, Vrbin CM, Geisinger KR. Urine cytology discrepancies: frequency, causes, and outcomes. Am J Clin Pathol. 2007; 127:946–53.6. Brimo F, Vollmer RT, Case B, Aprikian A, Kassouf W, Auger M. Accuracy of urine cytology and the significance of an atypical category. Am J Clin Pathol. 2009; 132:785–93.
Article7. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010; 140:883–99.
Article8. Sethi G, Shanmugam MK, Ramachandran L, Kumar AP, Tergaonkar V. Multifaceted link between cancer and inflammation. Biosci Rep. 2012; 32:1–15.
Article9. Zhang Y, Wang J, Xiang D, Wang D, Xin X. Alterations in the expression of the apurinic/apyrimidinic endonuclease-1/redox factor-1 (APE1/Ref-1) in human ovarian cancer and indentification of the therapeutic potential of APE1/Ref-1 inhibitor. Int J Oncol. 2009; 35:1069–79.
Article10. Park MS, Lee YR, Choi S, Joo HK, Cho EJ, Kim CS, et al. Identification of plasma APE1/Ref-1 in lipopolysaccharide-induced endotoxemic rats: implication of serological biomarker for an endotoxemia. Biochem Biophys Res Commun. 2013; 435:621–6.
Article11. Choi S, Lee YR, Park MS, Joo HK, Cho EJ, Kim HS, et al. Histone deacetylases inhibitor trichostatin A modulates the extracellular release of APE1/Ref-1. Biochem Biophys Res Commun. 2013; 435:403–7.
Article12. Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005; 7:367–84.
Article13. Tell G, Fantini D, Quadrifoglio F. Understanding different functions of mammalian AP endonuclease (APE1) as a promising tool for cancer treatment. Cell Mol Life Sci. 2010; 67:3589.
Article14. Puglisi F, Barbone F, Tell G, Aprile G, Pertoldi B, Raiti C, et al. Prognostic role of Ape/Ref-1 subcellular expression in stage I-III breast carcinomas. Oncol Rep. 2002; 9:11–7.
Article15. Dai N, Cao XJ, Li MX, Qing Y, Liao L, Lu XF, et al. Serum APE1 autoantibodies: a novel potential tumor marker and predictor of chemotherapeutic efficacy in non-small cell lung cancer. PLoS One. 2013; 8:e58001.
Article16. Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002; 87:13–5.17. Helpap B. New WHO classification of urothelial carcinoma of the urinary bladder. Verh Dtsch Ges Pathol. 2002; 86:57–66.18. Fluss R, Faraggi D, Reiser B. Estimation of the Youden Index and its associated cutoff point. Biom J. 2005; 47:458–72.
Article19. German RR, Fink AK, Heron M, Stewart SL, Johnson CJ, Finch JL, et al. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011; 35:126–31.
Article20. Offersen BV, Knap MM, Horsman MR, Verheijen J, Hanemaaijer R, Overgaard J. Matrix metalloproteinase-9 measured in urine from bladder cancer patients is an independent prognostic marker of poor survival. Acta Oncol. 2010; 49:1283–7.
Article21. Qu J, Liu GH, Huang B, Chen C. Nitric oxide controls nuclear export of APE1/Ref-1 through S-nitrosation of cysteines 93 and 310. Nucleic Acids Res. 2007; 35:2522–32.
Article22. Grossman HB, Messing E, Soloway M, Tomera K, Katz G, Berger Y, et al. Detection of bladder cancer using a point-of-care proteomic assay. JAMA. 2005; 293:810–6.
Article23. Huber S, Schwentner C, Taeger D, Pesch B, Nasterlack M, Leng G, et al. Nuclear matrix protein-22: a prospective evaluation in a population at risk for bladder cancer. Results from the UroScreen study. BJU Int. 2012; 110:699–708.
Article24. Lai Y, Ye J, Chen J, Zhang L, Wasi L, He Z, et al. UPK3A: a promising novel urinary marker for the detection of bladder cancer. Urology. 2010; 76:514.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Altered Secretory Activity of APE1/Ref-1 D148E Variants Identified in Human Patients With Bladder Cancer
- Dynamic Regulation of APE1/Ref-1 as a Therapeutic Target Protein
- Ape1/Ref-1 Stimulates GDNF/GFR alpha1-mediated Downstream Signaling and Neuroblastoma Proliferation
- Role of APE1/Ref-1 in hydrogen peroxide-induced apoptosis in human renal HK-2 cells
- APE1/Ref-1 as an emerging therapeutic target for various human diseases: phytochemical modulation of its functions