Cancer Res Treat.
2015 Oct;47(4):697-705. 10.4143/crt.2013.175.
Comparison of Surgery Plus Chemotherapy and Palliative Chemotherapy Alone for Advanced Gastric Cancer with Krukenberg Tumor
- Affiliations
-
- 1Division of Medical Oncology, Department of Internal Medicine, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. chojy@yuhs.ac
- 2Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2403388
- DOI: http://doi.org/10.4143/crt.2013.175
Abstract
- PURPOSE
This study was conducted to validate the survival benefit of metastasectomy plus chemotherapy over chemotherapy alone for treatment of Krukenberg tumors from gastric cancer and to identify prognostic factors for survival.
MATERIALS AND METHODS
Clinical data from 216 patients with Krukenberg tumors from gastric cancer were collected. Patients were divided into two arms according to treatment modality: arm A, metastasectomy plus chemotherapy and arm B, chemotherapy alone.
RESULTS
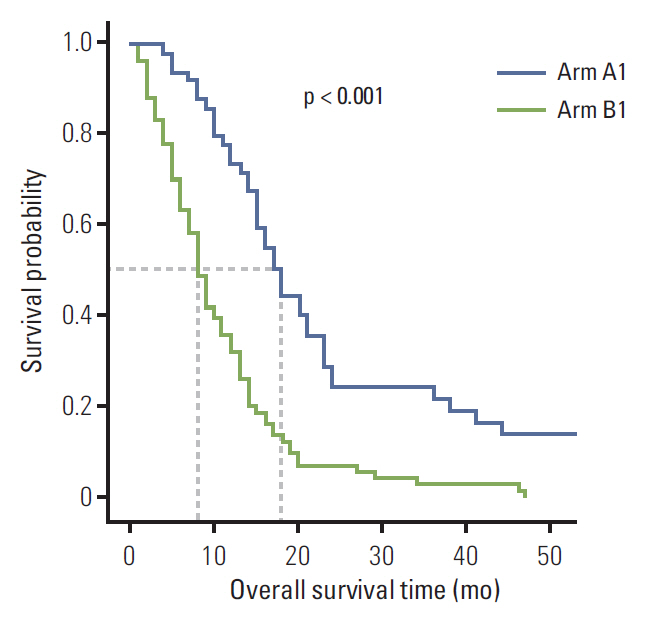
Overall survival (OS) was significantly increased in arm A relative to arm B for patients initially diagnosed with stage IV gastric cancer (18.0 months vs. 8.0 months; p < 0.001) and those with recurrent Krukenberg tumors (19.0 months vs. 9.0 months; p=0.002), respectively. Metastasectomy (hazard ratio [HR], 0.458; 95% confidence interval [CI], 0.287 to 0.732; p=0.001), signet-ring cell pathology (HR, 1.583; 95% CI, 1.057 to 2.371; p=0.026), and peritoneal carcinomatosis (HR, 3.081; 95% CI, 1.610 to 5.895; p=0.001) were significant prognostic factors for survival.
CONCLUSION
Metastasectomy plus chemotherapy offers superior OS when compared to palliative chemotherapy alone in gastric cancer with Krukenberg tumor. Prolonged survival applies to all patients, regardless of gastric cancer stage. Metastasectomy, signet-ring cell pathology, and peritoneal carcinomatosis were prognostic factors for survival. Future prospective randomized trials are needed to confirm the optimal treatment strategy for Krukenberg tumors from gastric cancer.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Wang J, Shi YK, Wu LY, Wang JW, Yang S, Yang JL, et al. Prognostic factors for ovarian metastases from primary gastric cancer. Int J Gynecol Cancer. 2008; 18:825–32.
Article2. Kim HK, Heo DS, Bang YJ, Kim NK. Prognostic factors of Krukenberg's tumor. Gynecol Oncol. 2001; 82:105–9.
Article3. Petru E, Pickel H, Heydarfadai M, Lahousen M, Haas J, Schaider H, et al. Nongenital cancers metastatic to the ovary. Gynecol Oncol. 1992; 44:83–6.
Article4. Hale RW. Krukenberg tumor of the ovaries: a review of 81 records. Obstet Gynecol. 1968; 32:221–5.5. Duarte I, Llanos O. Patterns of metastases in intestinal and diffuse types of carcinoma of the stomach. Hum Pathol. 1981; 12:237–42.
Article6. Saphir O. Signet-ring cell carcinoma. Mil Surg. 1951; 109:360–9.
Article7. Kim KH, Lee KW, Baek SK, Chang HJ, Kim YJ, Park DJ, et al. Survival benefit of gastrectomy ± metastasectomy in patients with metastatic gastric cancer receiving chemotherapy. Gastric Cancer. 2011; 14:130–8.8. Cheon SH, Rha SY, Jeung HC, Im CK, Kim SH, Kim HR, et al. Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol. 2008; 19:1146–53.
Article9. Elias D, Cavalcanti A, Sabourin JC, Lassau N, Pignon JP, Ducreux M, et al. Resection of liver metastases from colorectal cancer: the real impact of the surgical margin. Eur J Surg Oncol. 1998; 24:174–9.
Article10. Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996; 77:1254–62.11. Iwatsuki S, Esquivel CO, Gordon RD, Starzl TE. Liver resection for metastatic colorectal cancer. Surgery. 1986; 100:804–10.12. Imamura H, Matsuyama Y, Shimada R, Kubota M, Nakayama A, Kobayashi A, et al. A study of factors influencing prognosis after resection of hepatic metastases from colorectal and gastric carcinoma. Am J Gastroenterol. 2001; 96:3178–84.
Article13. Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. 2006; 30:277–99.14. Ekbom GA, Gleysteen JJ. Gastric malignancy: resection for palliation. Surgery. 1980; 88:476–81.15. Ayhan A, Guvenal T, Salman MC, Ozyuncu O, Sakinci M, Basaran M. The role of cytoreductive surgery in nongenital cancers metastatic to the ovaries. Gynecol Oncol. 2005; 98:235–41.
Article16. Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Gynecol Oncol. 1998; 69:103–8.
Article17. Zang RY, Zhang ZY, Li ZT, Chen J, Tang MQ, Liu Q, et al. Effect of cytoreductive surgery on survival of patients with recurrent epithelial ovarian cancer. J Surg Oncol. 2000; 75:24–30.
Article18. Kang SB, Park NH, Choi YM, Lee HP. Krukenberg tumors of the ovary: a clinical analysis of 16 cases. J Korean Cancer Assoc. 1990; 22:194–201.19. Lu LC, Shao YY, Hsu CH, Hsu C, Cheng WF, Lin YL, et al. Metastasectomy of Krukenberg tumors may be associated with survival benefits in patients with metastatic gastric cancer. Anticancer Res. 2012; 32:3397–401.20. Cheong JH, Hyung WJ, Chen J, Kim J, Choi SH, Noh SH. Survival benefit of metastasectomy for Krukenberg tumors from gastric cancer. Gynecol Oncol. 2004; 94:477–82.
Article21. Cheong JH, Hyung WJ, Chen J, Kim J, Choi SH, Noh SH. Surgical management and outcome of metachronous Krukenberg tumors from gastric cancer. J Surg Oncol. 2004; 87:39–45.
Article22. Nio Y, Tsubono M, Kawabata K, Masai Y, Hayashi H, Meyer C, et al. Comparison of survival curves of gastric cancer patients after surgery according to the UICC stage classification and the General Rules for Gastric Cancer Study by the Japanese Research Society for gastric cancer. Ann Surg. 1993; 218:47–53.
Article23. Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor: an overview. Arch Pathol Lab Med. 2006; 130:1725–30.
Article24. Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011; 17:1850–7.
Article25. Jacquet P, Jelinek JS, Steves MA, Sugarbaker PH. Evaluation of computed tomography in patients with peritoneal carcinomatosis. Cancer. 1993; 72:1631–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ERRATUM: Comparison of Surgery Plus Chemotherapy and Palliative Chemotherapy Alone for Advanced Gastric Cancer with Krukenberg Tumor
- Utility of Surgical Resection in the Management of Metachronous Krukenberg's Tumors of Gastric Origin
- Preoperative Chemotherapy in Advanced Stomach Cancer (Cons)
- A Case of Krukenberg Tumor associated with Ovarian Dermoid Cyst
- Efficacy and Safety Profile of TS-1 or TS-1/CDDP in Patients with Advanced Gastric Cancer