Ann Lab Med.
2018 Mar;38(2):147-154. 10.3343/alm.2018.38.2.147.
Clinical Implications of Quantitative JAK2 V617F Analysis using Droplet Digital PCR in Myeloproliferative Neoplasms
- Affiliations
-
- 1Center for Hematologic Malignancy, National Cancer Center, Goyang, Korea. hseom@ncc.re.kr ksy@ncc.re.kr
- 2Department of System Cancer Science, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea.
- 3Green Cross Laboratories, Yongin, Korea.
- 4Division of Oncology-Hematology, Department of Internal Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 5Department of Laboratory Medicine, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 6Department of Laboratory Medicine, National Cancer Center, Goyang, Korea.
- KMID: 2403360
- DOI: http://doi.org/10.3343/alm.2018.38.2.147
Abstract
- BACKGROUND
JAK2 V617F is the most common mutation in myeloproliferative neoplasms (MPNs) and is a major diagnostic criterion. Mutation quantification is useful for classifying patients with MPN into subgroups and for prognostic prediction. Droplet digital PCR (ddPCR) can provide accurate and reproducible quantitative analysis of DNA. This study was designed to verify the correlation of ddPCR with pyrosequencing results in the diagnosis of MPN and to investigate clinical implications of the mutational burden.
METHODS
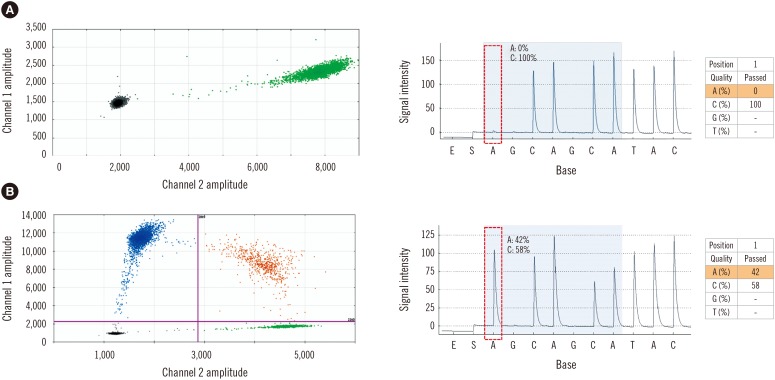
Peripheral blood or bone marrow samples were obtained from 56 patients newly diagnosed with MPN or previously diagnosed with MPN but not yet indicated for JAK2 inhibitor treatment between 2012 and 2016. The JAK2 V617F mutation was detected by pyrosequencing as a diagnostic work-up. The same samples were used for ddPCR to determine the correlation between assays and establish a detection sensitivity cut-off. Clinical and hematologic aspects were reviewed.
RESULTS
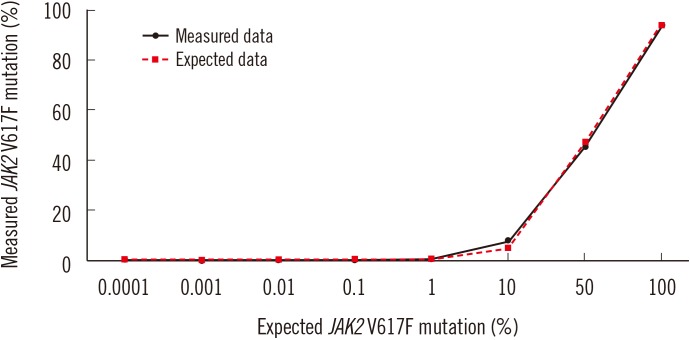
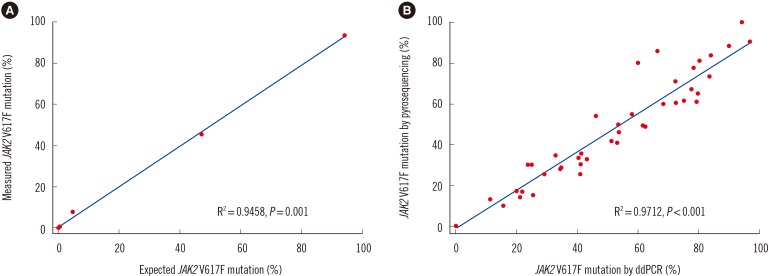
Forty-two (75%) and 46 (82.1%) patients were positive for JAK2 V617F by pyrosequencing and ddPCR, respectively. The mean mutated allele frequency at diagnosis was 37.5±30.1% and was 40.7±31.2% with ddPCR, representing a strong correlation (r=0.9712, P < 0.001). Follow-up samples were available for 12 patients, including eight that were JAK2 V617F-positive. Of these, mutational burden reduction after treatment was observed in six patients (75%), consistent with trends of hematologic improvement.
CONCLUSIONS
Quantitative analysis of the JAK2 V617F mutation using ddPCR was highly correlated with pyrosequencing data and may reflect the clinical response to treatment.
MeSH Terms
Figure
Reference
-
1. Swerdlow S, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein S, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC;2008.2. Cankovic M, Whiteley L, Hawley RC, Zarbo RJ, Chitale D. Clinical performance of JAK2 V617F mutation detection assays in a molecular diagnostics laboratory: evaluation of screening and quantitation methods. Am J Clin Pathol. 2009; 132:713–721. PMID: 19846812.3. Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR, et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med. 2005; 352:1779–1790. PMID: 15858187.4. Steensma DP, Dewald GW, Lasho TL, Powell HL, McClure RF, Levine RL, et al. The JAK2 V617F activating tyrosine kinase mutation is an infrequent event in both “atypical” myeloproliferative disorders and myelodysplastic syndromes. Blood. 2005; 106:1207–1209. PMID: 15860661.5. Busque L, Porwit A, Day R, Olney HJ, Leber B, Ethier V, et al. Laboratory investigation of myeloproliferative neoplasms (MPNs): recommendations of the Canadian Mpn Group. Am J Clin Pathol. 2016; 146:408–422. PMID: 27686169.6. Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127:2391–2405. PMID: 27069254.7. Passamonti F, Rumi E. Clinical relevance of JAK2 (V617F) mutant allele burden. Haematologica. 2009; 94:7–10. PMID: 19118374.8. Borowczyk M, Wojtaszewska M, Lewandowski K, Gil L, Lewandowska M, Lehmann-Kopydlowska A, et al. The JAK2 V617F mutational status and allele burden may be related with the risk of venous thromboembolic events in patients with Philadelphia-negative myeloproliferative neoplasms. Thromb Res. 2015; 135:272–280. PMID: 25559461.9. Campbell PJ, Griesshammer M, Dohner K, Dohner H, Kusec R, Hasselbalch HC, et al. V617F mutation in JAK2 is associated with poorer survival in idiopathic myelofibrosis. Blood. 2006; 107:2098–2100. PMID: 16293597.10. Koren-Michowitz M, Landman J, Cohen Y, Rahimi-Levene N, Salomon O, Michael M, et al. JAK2 V617F allele burden is associated with transformation to myelofibrosis. Leuk Lymphoma. 2012; 53:2210–2213. PMID: 22524513.11. Tefferi A, Lasho TL, Huang J, Finke C, Mesa RA, Li CY, et al. Low JAK2 V617F allele burden in primary myelofibrosis, compared to either a higher allele burden or unmutated status, is associated with inferior overall and leukemia-free survival. Leukemia. 2008; 22:756–761. PMID: 18216871.12. Kinz E, Leiherer A, Lang AH, Drexel H, Muendlein A. Accurate quantitation of JAK2 V617F allele burden by array-based digital PCR. Int J Lab Hematol. 2015; 37:217–224. PMID: 24963593.13. Bench AJ, White HE, Foroni L, Godfrey AL, Gerrard G, Akiki S, et al. Molecular diagnosis of the myeloproliferative neoplasms: UK guidelines for the detection of JAK2 V617F and other relevant mutations. Br J Haematol. 2013; 160:25–34. PMID: 23057517.14. Sanmamed MF, Fernandez-Landazuri S, Rodriguez C, Zarate R, Lozano MD, Zubiri L, et al. Quantitative cell-free circulating BRAF V600E mutation analysis by use of droplet digital PCR in the follow-up of patients with melanoma being treated with BRAF inhibitors. Clin Chem. 2015; 61:297–304. PMID: 25411185.15. Hindson BJ, Ness KD, Masquelier DA, Belgrader P, Heredia NJ, Makarewicz AJ, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011; 83:8604–8610. PMID: 22035192.16. Zhang Y, Xu Y, Zhong W, Zhao J, Chen M, Zhang L, et al. Total DNA input is a crucial determinant of the sensitivity of plasma cell-free DNA EGFR mutation detection using droplet digital PCR. Oncotarget. 2017; 8:5861–5873. PMID: 28052016.17. Otsuji K, Sasaki T, Tanaka A, Kunita A, Ikemura M, Matsusaka K, et al. Use of droplet digital PCR for quantitative and automatic analysis of the HER2 status in breast cancer patients. Breast Cancer Res Treat. 2017; 162:11–18. PMID: 28039535.18. Kim HR, Choi HJ, Kim YK, Kim HJ, Shin JH, Suh SP, et al. Allelic expression imbalance of JAK2 V617F mutation in BCR-ABL negative myeloproliferative neoplasms. PLoS One. 2013; 8:e52518. PMID: 23349688.19. Waterhouse M, Follo M, Pfeifer D, von Bubnoff N, Duyster J, Bertz H, et al. Sensitive and accurate quantification of JAK2 V617F mutation in chronic myeloproliferative neoplasms by droplet digital PCR. Ann Hematol. 2016; 95:739–744. PMID: 26931113.20. Reid AL, Freeman JB, Millward M, Ziman M, Gray ES. Detection of BRAF-V600E and V600K in melanoma circulating tumour cells by droplet digital PCR. Clin Biochem. 2015; 48:999–1002. PMID: 25523300.21. Watanabe M, Kawaguchi T, Isa S, Ando M, Tamiya A, Kubo A, et al. Ultra-sensitive detection of the pretreatment EGFR T790M mutation in non-small cell lung cancer patients with an EGFR-activating mutation using droplet digital PCR. Clin Cancer Res. 2015; 21:3552–3560. PMID: 25882755.22. Lange T, Edelmann A, Siebolts U, Krahl R, Nehring C, Jakel N, et al. JAK2 V617F allele burden in myeloproliferative neoplasms one month after allogeneic stem cell transplantation significantly predicts outcome and risk of relapse. Haematologica. 2013; 98:722–728. PMID: 23300178.23. Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. The Lancet. 2005; 365:1054–1061.24. Benati M, Montagnana M, Danese E, De Matteis G, Veneri D, Paviati E, et al. Role of JAK2 V617F mutation and aberrant expression of microRNA-143 in myeloproliferative neoplasms. Clin Chem Lab Med. 2015; 53:1005–1011. PMID: 25527813.25. Passamonti F, Rumi E, Pietra D, Elena C, Boveri E, Arcaini L, et al. A prospective study of 338 patients with polycythemia vera: the impact of JAK2 (V617F) allele burden and leukocytosis on fibrotic or leukemic disease transformation and vascular complications. Leukemia. 2010; 24:1574–1579. PMID: 20631743.26. Alshemmari SH, Rajaan R, Ameen R, Al-Drees MA, Almosailleakh MR. JAK2 V617F allele burden in patients with myeloproliferative neoplasms. Ann Hematol. 2014; 93:791–796. PMID: 24362471.27. Jones AV, Silver RT, Waghorn K, Curtis C, Kreil S, Zoi K, et al. Minimal molecular response in polycythemia vera patients treated with imatinib or interferon alpha. Blood. 2006; 107:3339–3341. PMID: 16352805.28. Vannucchi AM, Antonioli E, Guglielmelli P, Rambaldi A, Barosi G, Marchioli R, et al. Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood. 2007; 110:840–846. PMID: 17379742.29. Cervantes F, Vannucchi AM, Kiladjian JJ, Al-Ali HK, Sirulnik A, Stalbovskaya V, et al. Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood. 2013; 122:4047–4053. PMID: 24174625.30. Cervantes F, Pereira A. Does ruxolitinib prolong the survival of patients with myelofibrosis? Blood. 2017; 129:832–837. PMID: 28031182.31. Vannucchi AM, Kiladjian JJ, Griesshammer M, Masszi T, Durrant S, Passamonti F, et al. Ruxolitinib versus standard therapy for the treatment of polycythemia vera. N Engl J Med. 2015; 372:426–435. PMID: 25629741.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Real-time Semi-quantitative PCR, JAK2 MutaScreen (TM) Kit for JAK2 V617F Screening
- A Case of Acute Myeloid Leukemia Transformed from JAK2 V617F-Positive Chronic Neutrophilic Leukemia
- JAK2 V617F and the evolving paradigm of polycythemia vera
- Clinical features and outcomes of JAK2V617F-positive polycythemia vera and essential thrombocythemia according to the JAK2V617F allele burden
- The allele burden of JAK2 V617F can aid in differential diagnosis of Philadelphia Chromosome-Negative Myeloproliferative Neoplasm