Ann Lab Med.
2018 Mar;38(2):132-138. 10.3343/alm.2018.38.2.132.
Clinical and Cytogenetic Profiles of Rhabdomyosarcoma with Bone Marrow Involvement in Korean Children: A 15-Year Single-Institution Experience
- Affiliations
-
- 1Department of Laboratory Medicine, Gyeongsang National University Hospital, Jinju, Korea.
- 2Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea. ejseo@amc.seoul.kr
- 3Department of Pediatrics, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea.
- 4Department of Pathology, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea.
- KMID: 2403358
- DOI: http://doi.org/10.3343/alm.2018.38.2.132
Abstract
- BACKGROUND
Rhabdomyosarcoma (RMS) is the most common soft tissue sarcoma in children. Alveolar RMS (ARMS) is characterized by FOXO1-related chromosomal translocations that result in a poorer clinical outcome compared with embryonal RMS (ERMS). Because the chromosomal features of RMS have not been comprehensively defined, we analyzed the clinical and laboratory data of childhood RMS patients and determined the clinical significance of chromosomal abnormalities in the bone marrow.
METHODS
Fifty-one Korean patients with RMS < 18 years of age treated between 2001 and 2015 were enrolled in this study. Clinical factors, bone marrow and cytogenetic results, and overall survival (OS) were analyzed.
RESULTS
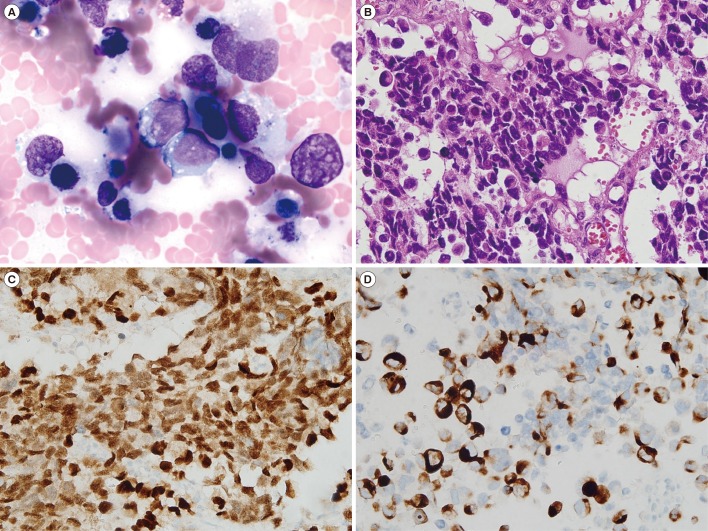
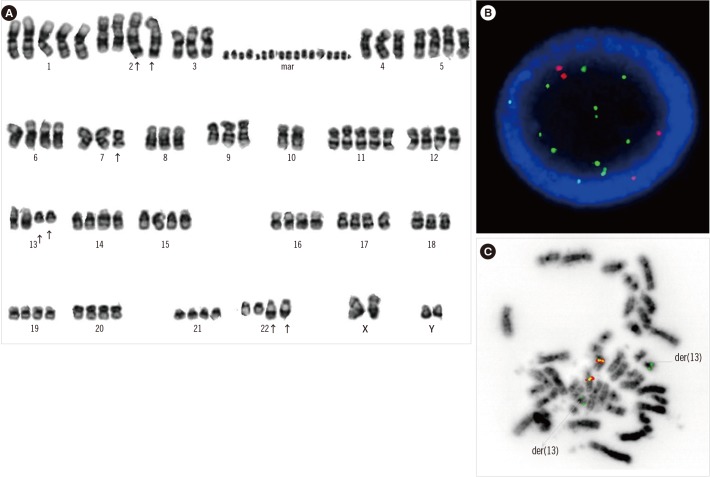
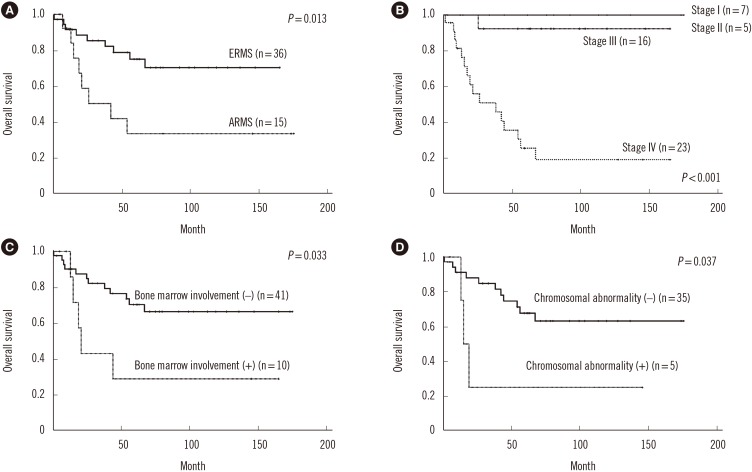
In total, 36 patients (70.6%) had ERMS and 15 (29.4%) had ARMS; 80% of the ARMS patients had stage IV disease. The incidences of bone and bone marrow metastases were 21.6% and 19.6%, respectively, and these results were higher than previously reported results. Of the 40 patients who underwent bone marrow cytogenetic investigation, five patients had chromosomal abnormalities associated with the 13q14 rearrangement. Patients with a chromosomal abnormality (15 vs 61 months, P=0.037) and bone marrow involvement (17 vs 61 months, P=0.033) had a significantly shorter median OS than those without such characteristics. Two novel rearrangements associated with the 13q14 locus were detected. One patient with concomitant MYCN amplification and PAX3/FOXO1 fusion showed an aggressive clinical course.
CONCLUSIONS
A comprehensive approach involving conventional cytogenetics and FOXO1 FISH of the bone marrow is needed to assess high-risk ARMS patients and identify novel cytogenetic findings.
MeSH Terms
Figure
Reference
-
1. Punyko JA, Mertens AC, Baker KS, Ness KK, Robison LL, Gurney JG. Long-term survival probabilities for childhood rhabdomyosarcoma. A population-based evaluation. Cancer. 2005; 103:1475–1483. PMID: 15712283.2. Barr FG, Duan F, Smith LM, Gustafson D, Pitts M, Hammond S, et al. Genomic and clinical analyses of 2p24 and 12q13-q14 amplification in alveolar rhabdomyosarcoma: a report from the Children's Oncology Group. Genes Chromosomes Cancer. 2009; 48:661–672. PMID: 19422036.3. Agamanolis DP, Dasu S, Krill CE Jr. Tumors of skeletal muscle. Hum Pathol. 1986; 17:778–795. PMID: 3525381.4. Sangkhathat S. Current management of pediatric soft tissue sarcomas. World J Clin Pediatr. 2015; 4:94–105. PMID: 26566481.5. Vleeshouwer-Neumann T, Phelps M, Bammler TK, MacDonald JW, Jenkins I, Chen EY. Histone deacetylase inhibitors antagonize distinct pathways to suppress tumorigenesis of embryonal rhabdomyosarcoma. PLoS One. 2015; 10:e0144320. PMID: 26636678.6. Goldstein M, Meller I, Issakov J, Orr-Urtreger A. Novel genes implicated in embryonal, alveolar, and pleomorphic rhabdomyosarcoma: a cytogenetic and molecular analysis of primary tumors. Neoplasia. 2006; 8:332–343. PMID: 16790082.7. Sorensen PH, Lynch JC, Qualman SJ, Tirabosco R, Lim JF, Maurer HM, et al. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the Children's Oncology Group. J Clin Oncol. 2002; 20:2672–2679. PMID: 12039929.8. Slater O, Shipley J. Clinical relevance of molecular genetics to paediatric sarcomas. J Clin Pathol. 2007; 60:1187–1194. PMID: 17468291.9. Krsková L, Mrhalová M, Hilská I, Sumerauer D, Drahokoupilová E, Múdry P, et al. Detection and clinical significance of bone marrow involvement in patients with rhabdomyosarcoma. Virchows Archiv. 2010; 456:463–472. PMID: 20405298.10. Keller C, Guttridge DC. Mechanisms of impaired differentiation in rhabdomyosarcoma. FEBS J. 2013; 280:4323–4334. PMID: 23822136.11. Sultan I, Qaddoumi I, Yaser S, Rodriguez-Galindo C, Ferrari A. Comparing adult and pediatric rhabdomyosarcoma in the surveillance, epidemiology and end results program, 1973 to 2005: an analysis of 2,600 patients. J Clin Oncol. 2009; 27:3391–3397. PMID: 19398574.12. Hosoi H, Teramukai S, Matsumoto Y, Tsuchiya K, Iehara T, Hara J, et al. A review of 331 rhabdomyosarcoma cases in patients treated between 1991 and 2002 in Japan. Int J Clin Oncol. 2007; 12:137–145. PMID: 17443282.13. Weiss AR, Lyden ER, Anderson JR, Hawkins DS, Spunt SL, Walterhouse DO, et al. Histologic and clinical characteristics can guide staging evaluations for children and adolescents with rhabdomyosarcoma: a report from the Children's Oncology Group Soft Tissue Sarcoma Committee. J Clin Oncol. 2013; 31:3226–3232. PMID: 23940218.14. Ognjanovic S, Linabery AM, Charbonneau B, Ross JA. Trends in childhood rhabdomyosarcoma incidence and survival in the United States, 1975-2005. Cancer. 2009; 115:4218–4226. PMID: 19536876.15. Schmitt-Ney M, Camussi G. The PAX3-FOXO1 fusion protein present in rhabdomyosarcoma interferes with normal FOXO activity and the TGF-β pathway. PLoS One. 2015; 10:e0121474. PMID: 25806826.16. Barr FG, Qualman SJ, Macris MH, Melnyk N, Lawlor ER, Strzelecki DM, et al. Genetic heterogeneity in the alveolar rhabdomyosarcoma subset without typical gene fusions. Cancer Res. 2002; 62:4704–4710. PMID: 12183429.17. Sumegi J, Streblow R, Frayer RW, Dal Cin P, Rosenberg A, Meloni-Ehrig A, et al. Recurrent t(2;2) and t(2;8) translocations in rhabdomyosarcoma without the canonical PAX-FOXO1 fuse PAX3 to members of the nuclear receptor transcriptional coactivator (NCOA) family. Genes Chromosomes Cancer. 2010; 49:224–236. PMID: 19953635.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rhabdomyosarcoma Presenting as Acute Leukemia Identified by Cytogenetic and FISH Analysis of Bone Marrow
- A Case of Rhabdomyosarcoma Involving Bone Marrow Confirmed by Immunohistochemical Staining
- Incidence and Histologic Patterns of Bone Marrow Involvement of Malignant Lymphoma Based on the World Health Organization Classification: A Single Institution Study
- Two Cases of Paratesticular Rhabdomyosarcoma
- Two Cases of Rhabdomyosarcoma of the Bladder in Children