J Pathol Transl Med.
2018 Jan;52(1):28-36. 10.4132/jptm.2017.09.25.
Reclassification of Mixed Oligoastrocytic Tumors Using a Genetically Integrated Diagnostic Approach
- Affiliations
-
- 1Department of Pathology, Seoul National University College of Medicine, Seoul, Korea. shparknp@snu.ac.kr
- 2Department of Neurosurgery, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Radiology, Seoul National University College of Medicine, Seoul, Korea.
- 4Department of Neurosicence Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2403255
- DOI: http://doi.org/10.4132/jptm.2017.09.25
Abstract
- BACKGROUND
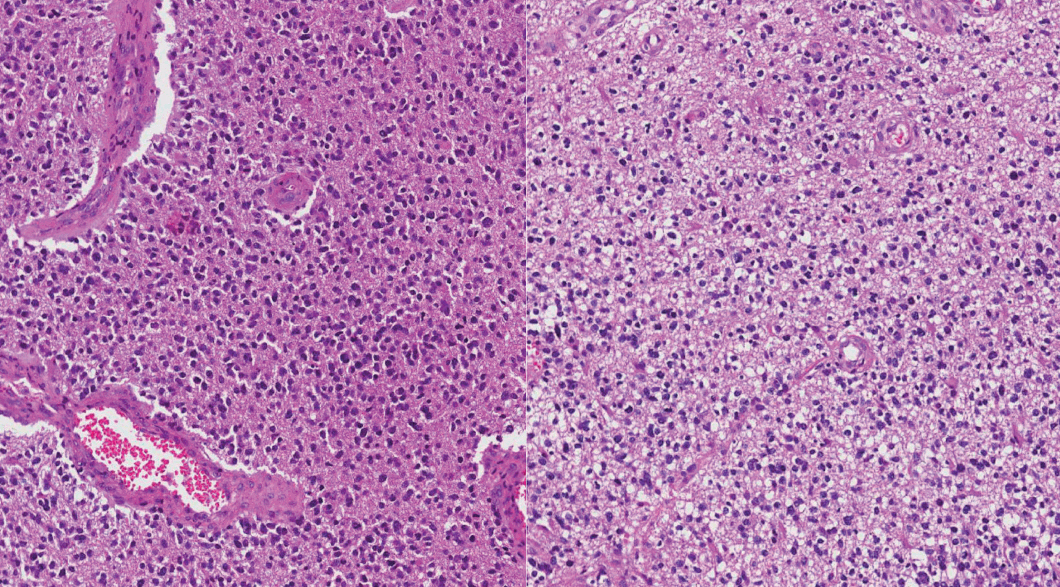
Mixed gliomas, such as oligoastrocytomas (OA), anaplastic oligoastrocytomas, and glioblastomas (GBMs) with an oligodendroglial component (GBMO) are defined as tumors composed of a mixture of two distinct neoplastic cell types, astrocytic and oligodendroglial. Recently, mutations ATRX and TP53, and codeletion of 1p/19q are shown to be genetic hallmarks of astrocytic and oligodendroglial tumors, respectively. Subsequent molecular analyses of mixed gliomas preferred the reclassification to either oligodendroglioma or astrocytoma. This study was designed to apply genetically integrated diagnostic criteria to mixed gliomas and determine usefulness and prognostic value of new classification in Korean patients.
METHODS
Fifty-eight cases of mixed OAs and GBMOs were retrieved from the pathology archives of Seoul National University Hospital from 2004 to 2015. Reclassification was performed according to genetic and immunohistochemical properties. Clinicopathological characteristics of each subgroup were evaluated. Overall survival was assessed and compared between subgroups.
RESULTS
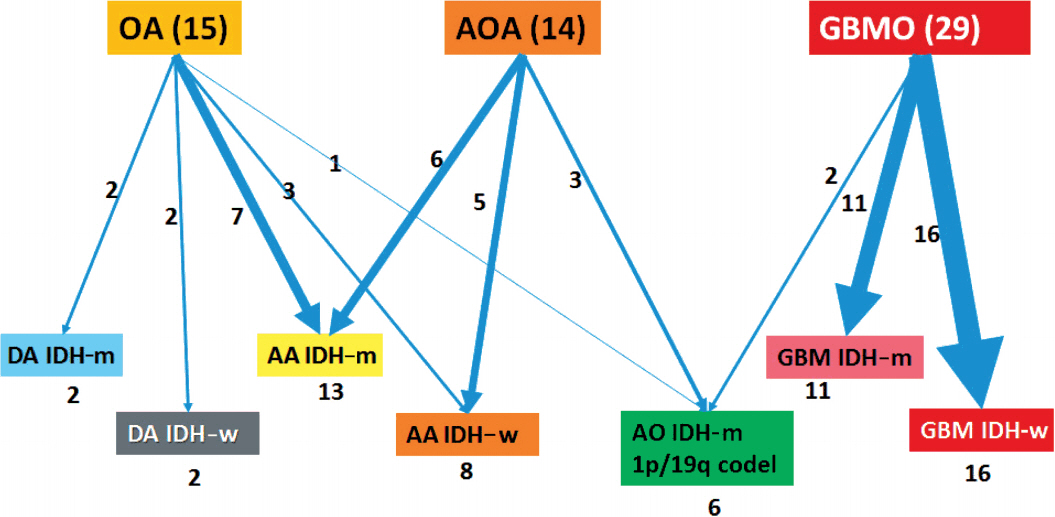
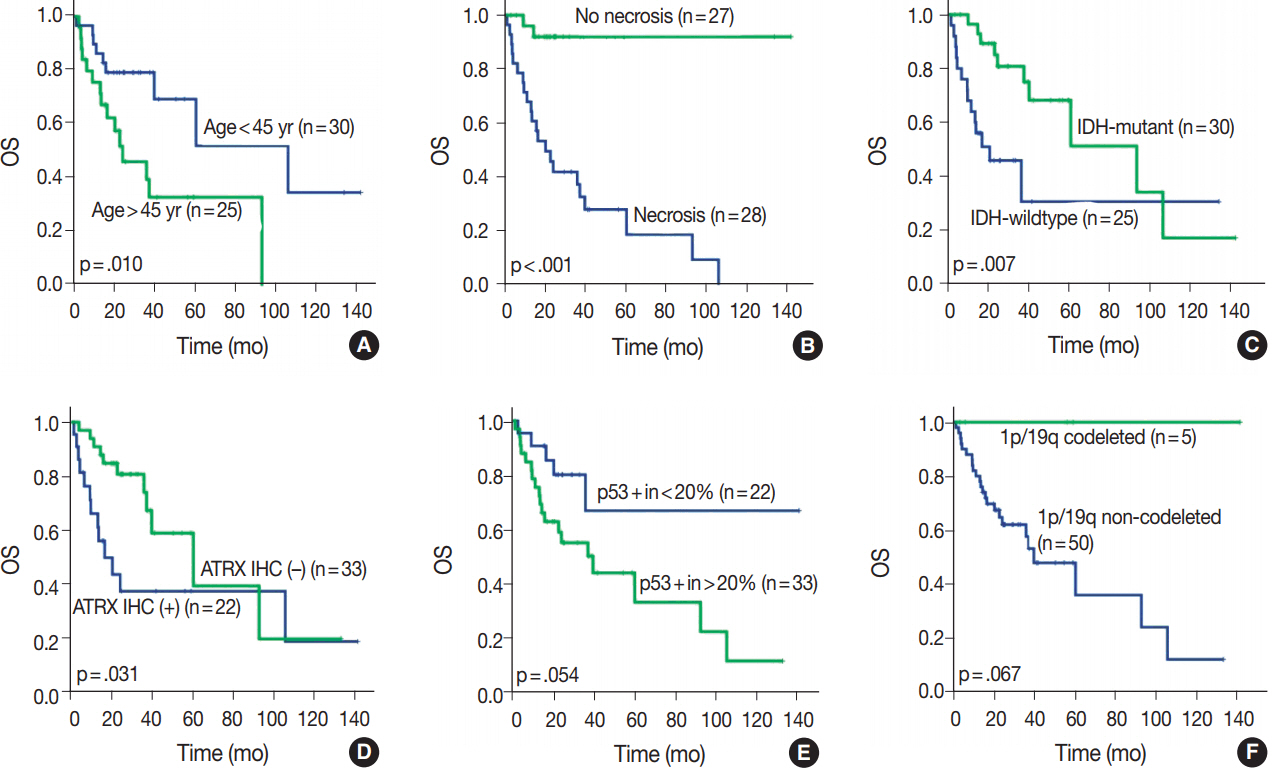
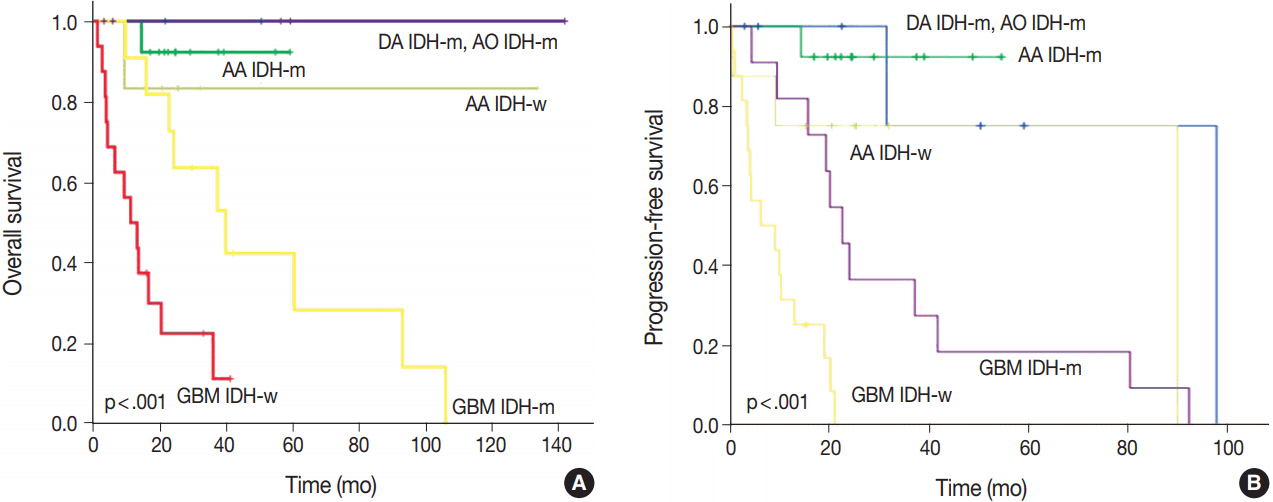
We could reclassify all mixed OAs and GBMOs into either astrocytic or oligodendroglial tumors. Notably, 29 GBMOs could be reclassified into 11 cases of GBM, IDH-mutant, 16 cases of GBM, IDH-wildtype, and two cases of anaplastic oligodendroglioma, IDH mutant. Overall survival was significantly different among these new groups (p<.001). Overall survival and progression-free survival were statistically better in gliomas with IDH mutation, ATRX mutation, no microscopic necrosis, and young patient age (cut off, 45 years old).
CONCLUSIONS
Our results strongly suggest that a genetically integrated diagnosis of glioma better reflects prognosis than former morphology-based methods.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The prognostic significance of p16 expression pattern in diffuse gliomas
Jin Woo Park, Jeongwan Kang, Ka Young Lim, Hyunhee Kim, Seong-Ik Kim, Jae Kyung Won, Chul-Kee Park, Sung-Hye Park
J Pathol Transl Med. 2021;55(2):102-111. doi: 10.4132/jptm.2020.10.22.
Reference
-
1. Myung JK, Cho HJ, Kim H, et al. Prognosis of glioblastoma with oligodendroglioma component is associated with the IDH1 mutation and MGMT methylation status. Transl Oncol. 2014; 7:712–9.2. Jiang H, Ren X, Cui X, et al. 1p/19q codeletion and IDH1/2 mutation identified a subtype of anaplastic oligoastrocytomas with prognosis as favorable as anaplastic oligodendrogliomas. Neuro Oncol. 2013; 15:775–82.3. Tortosa A, Viñolas N, Villà S, et al. Prognostic implication of clinical, radiologic, and pathologic features in patients with anaplastic gliomas. Cancer. 2003; 97:1063–71.
Article4. Okamoto Y, Di Patre PL, Burkhard C, et al. Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol. 2004; 108:49–56.
Article5. Ohgaki H. Contribution of molecular biology to the classification of low-grade diffuse glioma. In: Duffau H, ed. Diffuse low-grade gliomas in adults. London: Springer;2013. p. 61–72.6. Kros JM, Gorlia T, Kouwenhoven MC, et al. Panel review of anaplastic oligodendroglioma from European Organization For Research and Treatment of Cancer Trial 26951: assessment of consensus in diagnosis, influence of 1p/19q loss, and correlations with outcome. J Neuropathol Exp Neurol. 2007; 66:545–51.7. Killela PJ, Pirozzi CJ, Reitman ZJ, et al. The genetic landscape of anaplastic astrocytoma. Oncotarget. 2014; 5:1452–7.
Article8. Liu XY, Gerges N, Korshunov A, et al. Frequent ATRX mutations and loss of expression in adult diffuse astrocytic tumors carrying IDH1/IDH2 and TP53 mutations. Acta Neuropathol. 2012; 124:615–25.9. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO classification of tumours of the central nervous system. 4th ed. Lyon: IARC Press;2016.10. Labussière M, Idbaih A, Wang XW, et al. All the 1p19q codeleted gliomas are mutated on IDH1 or IDH2. Neurology. 2010; 74:1886–90.11. Louis DN, Perry A, Burger P, et al. International Society of Neuropathology: Haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 2014; 24:429–35.12. Sahm F, Reuss D, Koelsche C, et al. Farewell to oligoastrocytoma: in situ molecular genetics favor classification as either oligodendroglioma or astrocytoma. Acta Neuropathol. 2014; 128:551–9.13. Reuss DE, Sahm F, Schrimpf D, et al. ATRX and IDH1-R132H immunohistochemistry with subsequent copy number analysis and IDH sequencing as a basis for an “integrated” diagnostic approach for adult astrocytoma, oligodendroglioma and glioblastoma. Acta Neuropathol. 2015; 129:133–46.
Article14. van den Bent MJ, Brandes AA, Taphoorn MJ, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013; 31:344–50.15. Houillier C, Wang X, Kaloshi G, et al. IDH1 or IDH2 mutations predict longer survival and response to temozolomide in lowgrade gliomas. Neurology. 2010; 75:1560–6.16. Grisold W, Oberndorfer S, Struhal W. Stroke and cancer: a review. Acta Neurol Scand. 2009; 119:1–16.
Article17. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360:765–73.18. Haberler C, Wöhrer A. Clinical neuropathology practice news 2-2014: ATRX, a new candidate biomarker in gliomas. Clin Neuropathol. 2014; 33:108–11.
Article19. Love S, Perry A, Ironside J, Budka H. Greenfield's neuropathology. 9th ed. Boca Raton: CRC Press;2015.20. Louis DN, von Deimling A, Chung RY, et al. Comparative study of p53 gene and protein alterations in human astrocytic tumors. J Neuropathol Exp Neurol. 1993; 52:31–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Integrated diagnostic approach of pediatric neuromuscular disorders
- Long-Term Results of Intraoral Excision for Submandibular Mixed Tumors
- Transcranial Orbitotomy for Retroorbital Tumors
- Two Case of Malignant Mixed Mullerian Tumors
- Subungal Mixed Tumor Mimicking Glomus Tumor: A Case Report and Literature Review