J Educ Eval Health Prof.
2015;12:28. 10.3352/jeehp.2015.12.28.
Comparison of the knowledge, attitudes, and perception of barriers regarding adverse drug reaction reporting between pharmacy and medical students in Pakistan
- Affiliations
-
- 1Department of Clinical Pharmacy, Faculty of Pharmaceutical Sciences, UCSI University, Kuala Lumpur, Malaysia. akram@ucsiuniversity.edu.my
- 2Department of Paediatrics, Civil Hospital, Karachi, Pakistan.
- 3Faculty of Pharmacy, Hamdard University, Karachi, Pakistan.
- 4Dow Medical College, Dow University of Health Sciences, Karachi, Pakistan.
- 5Department of Pharmacy Practice, Kulliyyah of Pharmacy, International Islamic University Malaysia, Kuantan Campus, Kuantan, Malaysia.
- KMID: 2402035
- DOI: http://doi.org/10.3352/jeehp.2015.12.28
Abstract
- PURPOSE
The goal of this study was to compare the knowledge and attitudes of pharmacy and medical students regarding adverse drug reactions (ADRs), as well as their perceptions of barriers to ADR reporting, in a Higher Education Commission-recognised Pakistani university.
METHODS
A cross-sectional study was conducted among final-year pharmacy (n=91) and medical (n=108) students in Pakistan from June 1 to July 31, 2014. A self-administered questionnaire was used to collect the data. The responses of pharmacy students were compared to those of medical students.
RESULTS
Pharmacy students had a significantly better knowledge of ADRs than medical students (mean+/-SD, 5.61+/-1.78 vs. 3.23+/-1.60; P<0.001). Gender showed a significant relationship to knowledge about ADRs, and male participants were apparently more knowledgeable than their female counterparts (P<0.001). The attitudes of pharmacy students regarding their capability to handle and report ADRs were significantly more positive than those of medical students (P<0.05). In comparison to pharmacy students, a lack of knowledge of where and how to report ADRs was the main barrier that medical students perceived to ADR reporting (P=0.001).
CONCLUSION
Final-year pharmacy students exhibited more knowledge about ADRs and showed more positive attitudes regarding their capacity to handle and report ADRs than final-year medical students.
Keyword
MeSH Terms
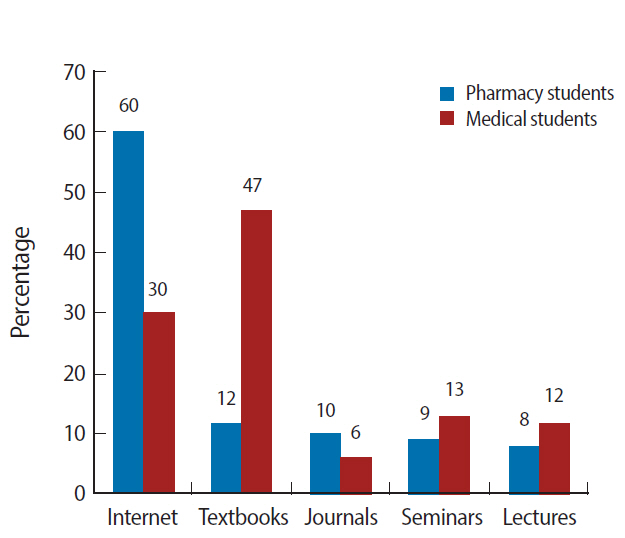
Figure
Cited by 1 articles
-
Palestinian pharmacists’ knowledge of issues related to using psychotropic medications in older people: a cross-sectional study
Ramzi Shawahna, Mais Khaskiyyi, Hadeel Abdo, Yasmen Msarwe, Rania Odeh, Souad Salame, Sun Huh
J Educ Eval Health Prof. 2017;14:8. doi: 10.3352/jeehp.2017.14.8.
Reference
-
1. Mahmood KT, Tahir FA, Haq IU. Pharmacovigilance: a need for best patient care in Pakistan: a review. J Pharm Sci Res. 2011; 3:1566–1584.2. Iffat W, Shakeel S, Naseem S, Imam S, Khan M. Attitudinal survey to assess medical and dental students’ belief of ADR reporting in Pakistan. Int J Pharm Pharm Sci. 2014; 6:279–283.3. Ahmad A, Patel I, Balkrishnan R, Mohanta GP, Manna PK. An evaluation of knowledge, attitude and practice of Indian pharmacists towards adverse drug reaction reporting: a pilot study. PerspectClin Res. 2013; 4:204–210. http://dx.doi.org/10.4103/2229-3485.120168.
Article4. Elkalmi RM, Hassali MA, Ibrahim MI, Widodo RT, Efan QM, Hadi MA. Pharmacy students’ knowledge and perceptions about pharmacovigilance in Malaysian public universities. Am J Pharm Educ. 2011; 75:96. http://dx.doi.org/10.5688/ajpe75596.
Article5. Sathvik BS, Chukir DM, Abo-Aldan E, Soliman MN. Adverse drug reaction monitoring and reporting: knowledge, attitude and belief of physicians & pharmacists of Aas al Khaimah, United Arab Emirates (UAE). Int J Pharm Sci Res. 2014; 5:368–375. http://dx.doi.org/10.13040/IJPSR.0975-8232.5(2).368-75.6. Li Q, Zhang SM, Chen HT, Fang SP, Yu X, Liu D, Shi LY, Zeng FD. Awareness and attitudes of healthcare professionals in Wuhan, China to the reporting of adverse drug reactions. Chin Med J (Engl). 2004; 117:856–861.7. Mann RD, Andrews EB. Pharmacovigilance. 2nd ed. West Sussex: John Wiley and Sons;2007.8. Granas AG, Buajordet M, Stenberg-Nilsen H, Harg P, Horn AM. Pharmacists’ attitudes towards the reporting of suspected adverse drug reactions in Norway. Pharmacoepidem Drug Safe. 2007; 16:429–434. http://dx.doi.org/10.1002/pds.1298.
Article9. Tabali M, Jeschke E, Bockelbrink A, Witt CM, Willich SN, Ostermann T, Matthes H. Educational intervention to improve physician reporting of adverse drug reactions (ADRs) in a primary care setting in complementary and alternative medicine. BMC Public Health. 2009; 9:274. http://dx.doi.org/10.1186/1471-2458-9-274.
Article10. Khan MU, Khan AN, Ahmed FR, Feroz Z, Rizvi SA, Shah S, Hussain R, Adil Z. Patients’ opinion of pharmacists and their roles in health care system in Pakistan. J Young Pharm. 2013; 5:90–94. http://dx.doi.org/10.1016/j.jyp.2013.08.001.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Investigation on Perceptions, Attitudes, and Contributing Factors to Spontaneous Adverse Drug Reaction Reporting among Community Pharmacists: Results from a Web-based Survey
- Impact of Safety Climate Perception and Barriers to Adverse Drug Reaction Reporting on Clinical Nurses' Monitoring Practice for Adverse Drug Reactions
- Influencing Factors of Clinical Nurses' Knowledge of Child Abuse Reporting, Perception of Child Abuse, and Moral Sensitivity on the Attitude toward Reporting Child Abuse
- Evaluation of Pharmacy Students' Perception on Clinical Pharmacy Practice Experience in the Tertiary and Secondary Hospital settings
- Effects of Awareness to Well-dying, Knowledge and Attitudes toward Advance-directives on Attitude toward End-of-life Care in Nursing Students