J Cerebrovasc Endovasc Neurosurg.
2017 Dec;19(4):291-300. 10.7461/jcen.2017.19.4.291.
Usefulness of Plaque Magnetic Resonance Imaging in Identifying High-Risk Carotid Plaques Irrespective of the Degree of Stenosis
- Affiliations
-
- 1Institute of Wonkwang Medical Science, Department of Neurosurgery, Wonkwang University School of Medicine, Iksan, Korea. kangsd@wku.ac.kr
- KMID: 2401782
- DOI: http://doi.org/10.7461/jcen.2017.19.4.291
Abstract
OBJECTIVE
Measurement of the degree of stenosis is not enough to decide on the treatment strategy for patients with carotid stenosis. Plaque morphology examination is needed for such a decision-making. Thus, we evaluated the usefulness of plaque magnetic resonance imaging (MRI) to decide on the modality of treatment for patients with carotid atherosclerotic plaques.
MATERIALS AND METHODS
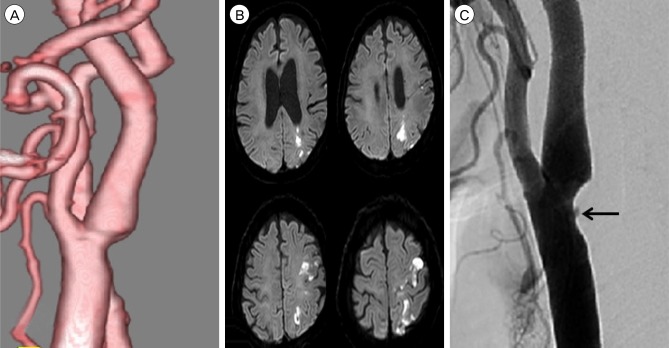
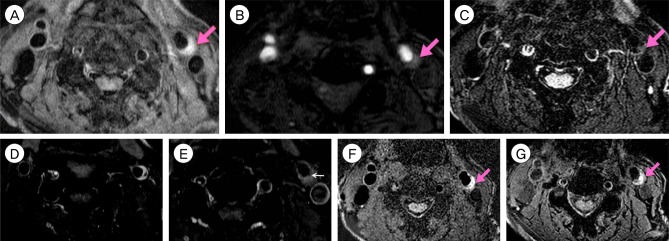
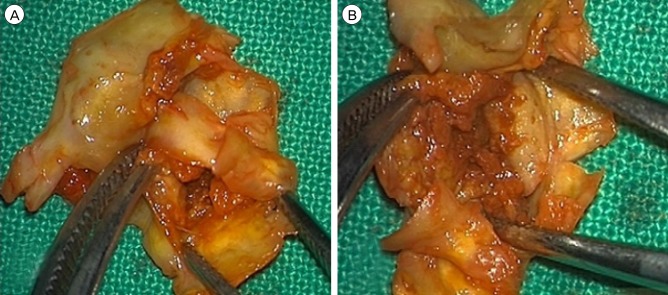
Fifteen patients presenting with carotid stenosis between 2014 and 2016 were included. They underwent angiography for measurement of the degree of stenosis. Carotid plaques were visualized using MRI.
RESULTS
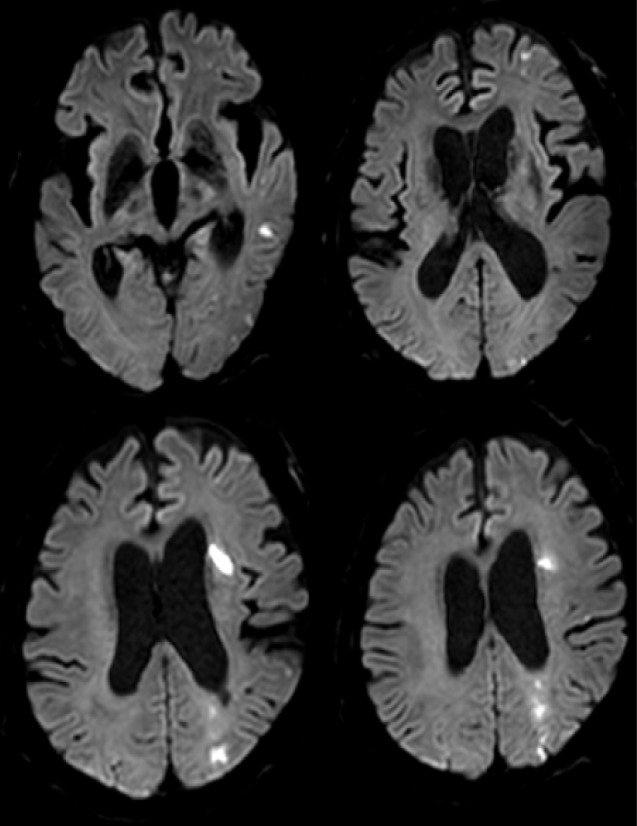
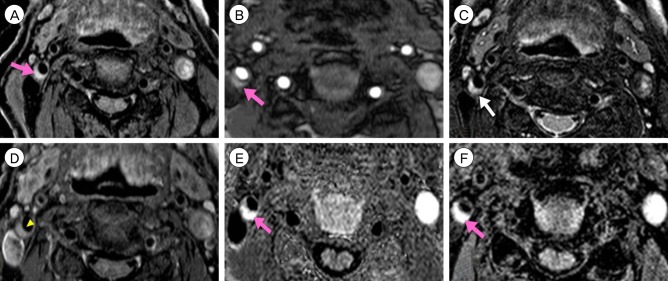
There were six (40%) stable and nine (60%) unstable plaques. Seven symptomatic patients (77.7%) had unstable lesions and two symptomatic patients (33.3%) had stable lesions (p = 0.096). There were six (40%) intraplaque hemorrhage (IPH) cases. There were six symptomatic patients (100%) in the IPH group and three symptomatic patients (33.3%) in the non-IPH group (p = 0.013). The mean stenosis degree was 58.9% in the IPH group and 70.4% in the non-IPH group (p = 0.094). Symptoms occurred irrespective of the degree of the stenosis in the IPH groups. In the IPH group, the recurrent ischemic cerebrovascular event rate was 33.3%. Particularly, the recurrent ischemic cerebrovascular event rate was 66.7% in the IPH group with mild stenosis treated with medications.
CONCLUSION
IPH in plaque MRI is significantly associated with ischemic symptoms and has a high risk for subsequent ischemic cerebrovascular events irrespective of the degree of stenosis. Plaque MRI is a useful tool in predicting symptomatic risks for carotid stenosis irrespective of the degree of such stenosis.
MeSH Terms
Figure
Reference
-
1. Altaf N, Daniels L, Morgan PS, Auer D, MacSweeney ST, Moody AR, et al. Detection of intraplaque hemorrhage by magnetic resonance imaging in symptomatic patients with mild to moderate carotid stenosis predicts recurrent neurological events. J Vasc Surg. 2008; 2. 47(2):337–342. PMID: 18164171.
Article2. Ambrose JA, Tannenbaum MA, Alexopoulos D, Hjemdahl-Monsen CE, Leavy J, Weiss M, et al. Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol. 1988; 7. 12(1):56–62. PMID: 3379219.
Article3. Bassiouny HS, Sakaguchi Y, Mikucki SA, McKinsey JF, Piano G, Gewertz BL, et al. Juxtalumenal location of plaque necrosis and neoformation in symptomatic carotid stenosis. J Vasc Surg. 1997; 10. 26(4):585–594. PMID: 9357458.
Article4. Cai JM, Hatsukami TS, Ferguson MS, Small R, Polissar NL, Yuan C. Classification of human carotid atherosclerotic lesions with in vivo multicontrast magnetic resonance imaging. Circulation. 2002; 9. 106(11):1368–1373. PMID: 12221054.
Article5. Carr S, Farb A, Pearce WH, Virmani R, Yao JS. Atherosclerotic plaque rupture in symptomatic carotid artery stenosis. J Vasc Surg. 1996; 5. 23(5):755–765. discussion 765-6. PMID: 8667496.
Article6. Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O'Brien KD, et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke. 2004; 5. 35(5):1079–1084. PMID: 15060318.
Article7. Esposito-Bauer L, Saam T, Ghodrati I, Pelisek J, Heider P, Bauer M, et al. MRI plaque imaging detects carotid plaques with a high risk for future cerebrovascular events in asymptomatic patients. PLoS One. 2013; 7. 8(7):e67927. PMID: 23894291.
Article8. Falk E. Why do plaques rupture? Circulation. 1992; 12. 86(6 Suppl):III30–III42. PMID: 1424049.9. Golledge J, Greenhalgh RM, Davies AH. The symptomatic carotid plaque. Stroke. 2000; 3. 31(3):774–781. PMID: 10700518.
Article10. Lusby RJ, Ferrell LD, Ehrenfeld W, Stoney R, Wylie EJ. Carotid plaque hemorrhage: its role in production of cerebral ischemia. Arch Surg. 1982; 11. 117(11):1479–1488. PMID: 6182861.11. Maynor CH, Charles HC, Herfkens RJ, Suddarth SA, Johnson GA. Chemical shift imaging of atherosclerosis at 7.0 Tesla. Invest Radiol. 1989; 1. 24(1):52–60. PMID: 2917823.
Article12. Mofidi R, Crotty TB, McCarthy P, Sheehan SJ, Mehigan D, Keaveny TV. Association between plaque instability, angiogenesis and symptomatic carotid occlusive disease. Br J Surg. 2001; 7. 88(7):945–950. PMID: 11442525.
Article13. Robless P, Baxter A, Byrd S, Emson M, Halliday A. Prevalence of asymptomatic CT infarcts in the ongoing Asymptomatic Carotid Surgery Trial (ACST). Int Angiol. 1998; 9. 17(3):194–200. PMID: 9821034.14. Rothwell PM, Eliasziw M, Gutnikov SA, Fox AJ, Taylor DW, Mayberg MR, et al. Analysis of pooled data from the randomised controlled trials of endarterectomy for symptomatic carotid stenosis. Lancet. 2003; 1. 361(9352):107–116. PMID: 12531577.
Article15. Takaya N, Yuan C, Chu B, Saam T, Underhill H, Cai J, et al. Association between carotid plaque characteristics and subsequent ischemic cerebrovascular events: a prospective assessment with MRI--initial results. Stroke. 2006; 3. 37(3):818–823. PMID: 16469957.
Article16. Toussaint JF, LaMuraglia GM, Southern JF, Fuster V, Kantor HL. Magnetic resonance images lipid, fibrous, calcified, hemorrhagic, and thrombotic components of human atherosclerosis in vivo. Circulation. 1996; 9. 94(5):932–938. PMID: 8790028.
Article17. Toussaint JF, Southern JF, Fuster V, Kantor HL. T2-weighted contrast for NMR characterization of human atherosclerosis. Arterioscler Thromb Vasc Biol. 1995; 10. 15(10):1533–1542. PMID: 7583524.18. Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005; 10. 25(10):2054–2061. PMID: 16037567.19. Walker MD, Marler JR, Goldstein M, Grady PA, Toole JF, Baker WH, et al. Endarterectomy for asymptomatic carotid artery stenosis. JAMA. 1995; 5. 273(18):1421–1428. PMID: 7723155.
Article20. Yuan C, Hatsukami TS, Beach KW, Hayes CE, Nelson JA, Ferguson MS, et al. In vivo MR evaluation of atherosclerosis in human carotid artery with use of phased-array coils. J Vasc Interv Radiol. 1996; Jan-Feb. 7(1):46–48.
Article21. Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001; 10. 104(17):2051–2056. PMID: 11673345.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High-Resolusion Magnetic Resonance Imaging of Carotid Atherosclerotic Plaque
- Carotid Intraplaque Hemorrhage is Associated with Acute Cerebral Ischemic Events and Progression of Stenosis on Magnetic Resonance Imaging
- Usefulness of carotid ultrasonography and treatment of carotid disease
- Assessment of Carotid Diffusion-Weighted Imaging for Detection of Lipid-Rich Necrotic Core in Symptomatic Carotid Atheroma
- Noninvasive Imaging of Atherosclerotic Plaques Using MRI and CT