J Lipid Atheroscler.
2017 Dec;6(2):89-96. 10.12997/jla.2017.6.2.89.
Effect of Long-term Fenofibrate Therapy on Serum Creatinine and Its Reversibility in Hypertriglyceridemic Patients with Hypertension
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, College of Medicine, Chung-Ang University, Seoul, Korea. cjkim@cau.ac.kr
- KMID: 2401111
- DOI: http://doi.org/10.12997/jla.2017.6.2.89
Abstract
OBJECTIVE
Previous studies have shown that fenofibrate therapy increases serum creatinine level and that there is a return of serum creatinine to baseline level after the discontinuation of the drug. We evaluated the effect of long-term fenofibrate therapy on creatinine levels and its reversibility in patients with hypertension and hypertriglyceridemia.
METHODS
This retrospective study enrolled 54 hypertensive and hypertriglyceridemic patients taking fenofibrate for 3-6 years (Fenofibrate group) and 30 control patients with similar age, sex, follow-up duration, and creatinine levels (Control group). In 23 patients taking fenofibrate with low triglyceride level and/or with high creatinine levels, fenofibrate was discontinued, and creatinine levels were measured after 2 months.
RESULTS
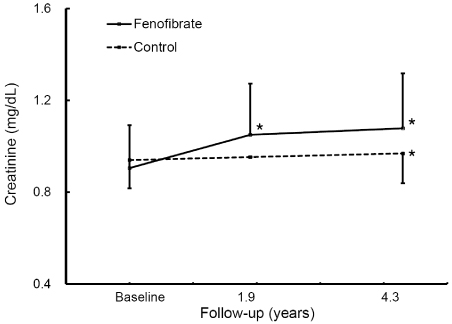
Creatinine levels increased in both the fenofibrate group (from 0.91±0.18 mg/dL to 1.09±0.23 mg/dL, p < 0.001) and the control group (from 0.94±0.16 mg/dL to 0.98±0.16 mg/dL, p=0.04) compared to baseline. However, the elevation was more pronounced in the fenofibrate group than in the control group (21.1±15.4% vs. 4.5±11.3%, p < 0.001). The discontinuation of fenofibrate lowered creatinine levels (from 1.39±0.32 mg/dL to 1.15±0.24 mg/dL, p < 0.001) which were still higher than pre-treatment levels (p=0.013).
CONCLUSION
Long-term fenofibrate therapy significantly increased creatinine levels in hypertensive and hypertriglyceridemic patients. The effect of fenofibrate on creatinine level was partially reversible. This finding suggests that follow-up creatinine level is necessary with fenofibrate therapy.
MeSH Terms
Figure
Reference
-
1. Shah A, Rader DJ, Millar JS. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis. 2010; 210:35–40.2. Kim CJ. Management of hypertriglyceridemia for prevention of cardiovascular diseases. J Lipid Atheroscler. 2013; 2:53–60.
Article3. Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol. 2007; 99:3C–18C.
Article4. Lageder H. Comparative double-blind investigation of bezafibrate and clofibrate in patients with primary hyperlipoproteinaemia. Wien Klin Wochenschr. 1980; 92:95–101.5. Dick TB, Marples J, Ledermann HM, Whittington J. Comparative study of once and 3-times daily regimens of bezafibrate in patients with primary hyperlipoproteinaemia. Curr Med Res Opin. 1981; 7:489–502.
Article6. Rössner S, Orö L. Fenofibrate therapy of hyperlipoproteinaemia. A dose-response study and a comparison with clofibrate. Atherosclerosis. 1981; 38:273–282.
Article7. Diabetes Atherosclerosis Intervention Study Investigators. Effect of fenofibrate on progression of coronaryartery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001; 357:905–910.8. Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005; 366:1849–1861.9. ACCORD Study Group, Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362:1563–1574.
Article10. Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007; 370:591–603.
Article11. Hopkins PN, Hunt SC, Wu LL, Williams GH, Williams RR. Hypertension, dyslipidemia, and insulin resistance: links in a chain or spokes on a wheel. Curr Opin Lipidol. 1996; 47:241–253.
Article12. Broeders N, Knoop C, Antoine M, Tielemans C, Abramowicz D. Fibrate-induced increase in blood urea and creatinine: is gemfibrozil the only innocuous agent? Nephrol Dial Transplant. 2000; 15:1993–1999.
Article13. Hottelart C, El Esper N, Rose F, Achard JM, Fournier A. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron. 2002; 92:536–541.
Article14. Ritter JL, Nabulsi S. Fenofibrate-induced elevation in serum creatinine. Pharmacotherapy. 2001; 21:1145–1149.
Article15. Deighan CJ, Caslake MJ, McConnell M, Boulton-Jones JM, Packard CJ. Comparative effects of cerivastatin and fenofibrate on the atherogenic lipoprotein phenotype in proteinuric renal disease. J Am Soc Nephrol. 2001; 12:341–348.
Article16. Ansquer JC, Dalton RN, Caussé E, Crimet D, Le Malicot K, Foucher C. Effect of fenofibrate on kidney function: a 6-week randomized crossover trial in healthy people. Am J Kidney Dis. 2008; 51:904–913.
Article17. Brancati FL, Whelton PK, Randall BL, Neaton JD, Stamler J, Klag MJ. Multiple Risk Factor Intervention Trial. Risk of end-stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT. JAMA. 1997; 278:2069–2074.18. Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999; 341:1127–1133.
Article19. Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia. 2011; 54:280–290.
Article20. Williams AJ, Baker F, Walls J. The short term effects of bezafibrate on the hypertriglyceridaemia of moderate to severe uraemia. Br J Clin Pharmacol. 1984; 18:361–367.
Article21. Angeles C, Lane BP, Miller F, Nord EP. Fenofibrateassociated reversible acute allograft dysfunction in 3 renal transplant recipients: biopsy evidence of tubular toxicity. Am J Kidney Dis. 2004; 44:543–550.
Article22. Dierkes J, Westphal S, Luley C. Serum homocysteine increases after therapy with fenofibrate or bezafibrate. Lancet. 1999; 354:219–220.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Short-term Fenofibrate Therapy on Blood Creatinine Levels in Patients with Hypertriglyceridemia
- Effect of Fenofibrate Therapy on Blood Creatinine Levels in Patients with Hypertension and Hypertriglyceridemia
- Fenofibrate Therapy and Change of Renal Function: Management, What and How?
- Fenofibrate Reduces C-Reactive Protein Levels in Hypertriglyceridemic Patients With High Risks for Cardiovascular Diseases
- Effect of fenofibrate on C-reactive protein levels in hypertriglyceridemic patients