J Lipid Atheroscler.
2013 Jun;2(1):19-26. 10.12997/jla.2013.2.1.19.
Effect of Fenofibrate Therapy on Blood Creatinine Levels in Patients with Hypertension and Hypertriglyceridemia
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Chung-Ang University, Seoul, Korea. cjkim@cau.ac.kr
- KMID: 2095091
- DOI: http://doi.org/10.12997/jla.2013.2.1.19
Abstract
OBJECTIVE
Previous studies have reported that fenofibrate therapy increased blood creatinine levels. We investigated the effect of fenofibrate therapy on creatinine levels in patients with hypertension and hypertriglyceridemia.
METHODS
This retrospective study included 36 hypertensive patients with hypertriglyceridemia taking fenofibrate for 1-3 years (Fenofibrate group) and 36 control patients with similar age, sex, follow-up duration, creatinine levels, and lipid levels to those of fenofibrate therapy (Control group).
RESULTS
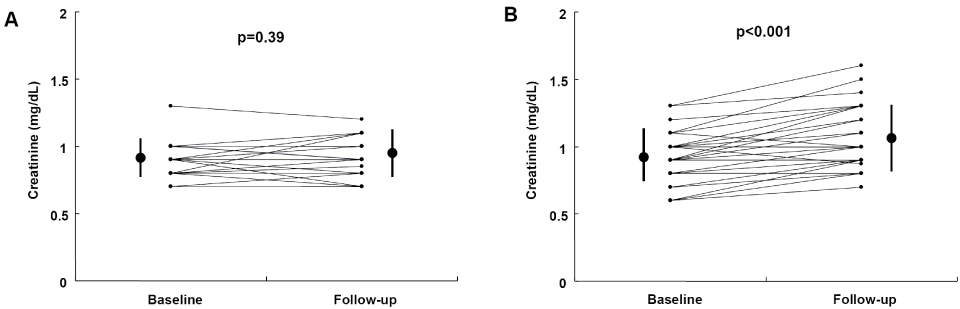
Baseline parameters except lipid profiles were similar between the fenofibrate and control groups. Creatinine levels increased in the fenofibrate group (from 0.90+/-0.18 mg/dL to 1.05+/-0.22 mg/dL, p<0.001) and did not change in the control group (from 0.91+/-0.12 mg/dL to 0.92+/-0.14 mg/dL, p=0.39). The elevation was more pronounced in the fenofibrate group than in the control group (0.15+/-0.12 vs. 0.02+/-0.11 mg/dL, p<0.001). Changes in creatinine levels were only associated with fenofibrate therapy (r=0.52, p<0.001) in the stepwise linear regression analysis.
CONCLUSION
Fenofibrate therapy for 1-3 years significantly increased creatinine levels in hypertensive patients with hypertriglyceridemia. This finding suggests that follow-up measurement of creatinine level is necessary with fenofibrate therapy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Management of Hypertriglyceridemia for Prevention of Cardiovascular Diseases
Chee Jeong Kim
J Lipid Atheroscler. 2013;2(2):53-60. doi: 10.12997/jla.2013.2.2.53.
Reference
-
1. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Cholesterol Treatment Trialists' (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005; 366:1267–1278.
Article2. Kim CJ, Lee KJ. Hypercholesterolemia; management of Korean patients in new millennium. Korean J Med. 2007; 72:580–592.3. Forrester JS, Libby P. The inflammation hypothesis and its potential relevance to statin therapy. Am J Cardiol. 2007; 99:732–738.
Article4. Cho JH, Kim KJ, Lee WS, Lee KJ, Kim SW, Kim TH, Kim CJ. Effect of statins on C-reactive protein, lipoprotein(a) and fibrinogen in hypercholesterolemic patients. J Lipid Atheroscler. 2012; 1:21–28.
Article5. Shah A, Rader DJ, Millar JS. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis. 2010; 210:35–40.
Article6. Abourbih S, Filion KB, Joseph L, Schiffrin EL, Rinfret S, Poirier P, Pilote L, Genest J, Eisenberg MJ. Effect of fibrates on lipid profiles and cardiovascular outcomes: a systematic review. Am J Med. 2009; 122:962.e1–962.e8.
Article7. Davidson MH, Armani A, McKenney JM, Jacobson TA. Safety considerations with fibrate therapy. Am J Cardiol. 2007; 99:3C–18C.
Article8. Lageder H. Comparative double-blind investigation of bezafibrate and clofibrate in patients with primary hyperlipoproteinaemia. Wien Klin Wochenschr. 1980; 92:95–101.9. Dick TB, Marples J, Ledermann HM, Whittington J. Comparative study of once and 3-times daily regimens of bezafibrate in patients with primary hyperlipoproteinaemia. Curr Med Res Opin. 1981; 7:489–502.
Article10. Rössner S, Orö L. Fenofibrate therapy of hyperlipoproteinaemia. A dose-response study and a comparison with clofibrate. Atherosclerosis. 1981; 38:273–282.
Article11. Diabetes Atherosclerosis Intervention Study Investigators. Effect of fenofibrate on progression of coronaryartery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001; 357:905–910.12. Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesäniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M. FIELD study investigators. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005; 366:1849–1861.
Article13. ACCORD Study Group. Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail-Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons-Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010; 362:1563–1574.14. Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007; 370:591–603.
Article15. Hopkins PN, Hunt SC, Wu LL, Williams GH, Williams RR. Hypertension, dyslipidemia, and insulin resistance: links in a chain or spokes on a wheel? Curr Opin Lipidol. 1996; 7:241–253.
Article16. Broeders N, Knoop C, Antoine M, Tielemans C, Abramowicz D. Fibrate-induced increase in blood urea and creatinine: is gemfibrozil the only innocuous agent? Nephrol Dial Transplant. 2000; 15:1993–1999.
Article17. Hottelart C, El Esper N, Rose F, Achard JM, Fournier A. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron. 2002; 92:536–541.
Article18. Ritter JL, Nabulsi S. Fenofibrate-induced elevation in serum creatinine. Pharmacotherapy. 2001; 21:1145–1149.
Article19. Deighan CJ, Caslake MJ, McConnell M, Boulton-Jones JM, Packard CJ. Comparative effects of cerivastatin and fenofibrate on the atherogenic lipoprotein phenotype in proteinuric renal disease. J Am Soc Nephrol. 2001; 12:341–348.
Article20. Ansquer JC, Dalton RN, Caussé E, Crimet D, Le Malicot K, Foucher C. Effect of fenofibrate on kidney function: a 6-week randomized crossover trial in healthy people. Am J Kidney Dis. 2008; 51:904–913.
Article21. Brancati FL, Whelton PK, Randall BL, Neaton JD, Stamler J, Klag MJ. Risk of end-stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT. Multiple Risk Factor Intervention Trial. JAMA. 1997; 278:2069–2074.
Article22. Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med. 1999; 341:1127–1133.
Article23. Davis TM, Ting R, Best JD, Donoghoe MW, Drury PL, Sullivan DR, Jenkins AJ, O'Connell RL, Whiting MJ, Glasziou PP, Simes RJ, Kesäniemi YA, Gebski VJ, Scott RS, Keech AC. Fenofibrate Intervention and Event Lowering in Diabetes Study investigators. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia. 2011; 54:280–290.
Article24. Williams AJ, Baker F, Walls J. The short term effects of bezafibrate on the hypertriglyceridaemia of moderate to severe uraemia. Br J Clin Pharmacol. 1984; 18:361–367.
Article25. Hottelart C, El Esper N, Rose F, Achard JM, Fournier A. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron. 2002; 92:536–541.
Article26. Angeles C, Lane BP, Miller F, Nord EP. Fenofibrate-associated reversible acute allograft dysfunction in 3 renal transplant recipients: biopsy evidence of tubular toxicity. Am J Kidney Dis. 2004; 44:543–550.
Article27. Dierkes J, Westphal S, Luley C. Serum homocysteine increases after therapy with fenofibrate or bezafibrate. Lancet. 1999; 354:219–220.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Long-term Fenofibrate Therapy on Serum Creatinine and Its Reversibility in Hypertriglyceridemic Patients with Hypertension
- Effect of Short-term Fenofibrate Therapy on Blood Creatinine Levels in Patients with Hypertriglyceridemia
- Fenofibrate Therapy and Change of Renal Function: Management, What and How?
- Effect of Fenofibrate Medication on Renal Function
- Effect of Fenofibrate in Combination with Urate Lowering Agents in Patients with Gout