Anat Cell Biol.
2017 Dec;50(4):284-292. 10.5115/acb.2017.50.4.284.
Pre-treated Populus tomentiglandulosa extract inhibits neuronal loss and alleviates gliosis in the gerbil hippocampal CA1 area induced by transient global cerebral ischemia
- Affiliations
-
- 1Department of Biomedical Science, Research Institute of Bioscience and Biotechnology, Hallym University, Chuncheon, Korea.
- 2Department of Neurobiology, Kangwon National University School of Medicine, Chuncheon, Korea. mhwon@kangwon.ac.kr
- 3Division of Food Biotechnology, School of Biotechnology, Kangwon National University, Chuncheon, Korea.
- 4Department of Emergency Medicine, Sooncheonhyang University Seoul Hospital, Sooncheonhyang University College of Medicine, Seoul, Korea.
- 5Department of Food Science and Nutrition, Hallym University, Chuncheon, Korea.
- 6Department of Surgery, Kangwon National University School of Medicine, Chuncheon, Korea.
- 7Department of Radiology, Kangwon National University School of Medicine, Chuncheon, Korea.
- 8Department of Physiology and Institute of Neurodegeneration and Neuroregeneration, Hallym University College of Medicine, Chuncheon, Korea. yylee@hallym.ac.kr
- KMID: 2399897
- DOI: http://doi.org/10.5115/acb.2017.50.4.284
Abstract
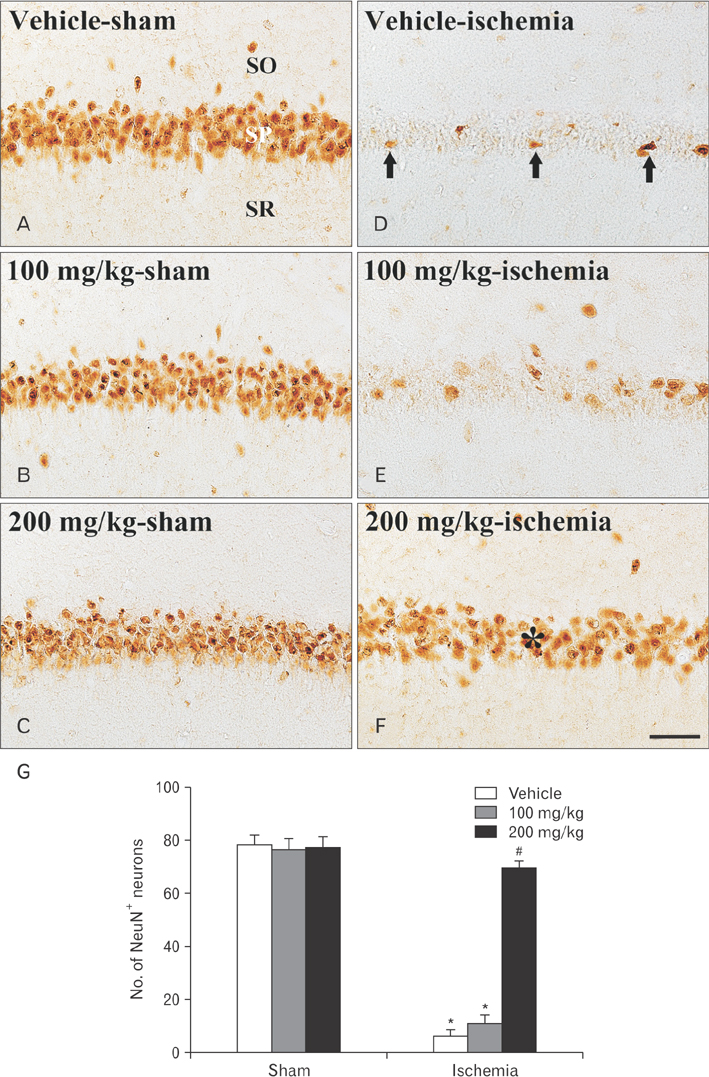
- The genus Populus (poplar) belonging to the Salicaceae family has been used in traditional medicine, and its several species show various pharmacological properties including antioxidant and anti-inflammatory effects. No study regarding protective effects of Populus species against cerebral ischemia has been reported. Therefore, in the present study, we examined neuroprotective effects of ethanol extract from Populus tomentiglandulosa (Korea poplar) in the hippocampal cornu ammonis (CA1) area of gerbils subjected to 5 minutes of transient global cerebral ischemia. Pretreatment with 200 mg/kg of P. tomentiglandulosa extract effectively protected CA1 pyramidal neurons from transient global cerebral ischemia. In addition, glial fibrillary acidic protein immunoreactive astrocytes and ionized calcium binding adapter molecule 1 immunoreactive microglia were significantly diminished in the ischemic CA1 area by pretreatment with 200 mg/kg of P. tomentiglandulosa extract. Briefly, our results indicate that pretreatment with P. tomentiglandulosa extract protects neurons from transient cerebral ischemic injury and diminish cerebral ischemia-induced reactive gliosis in ischemic CA1 area. Based on these results, we suggest that P. tomentiglandulosa can be used as a potential candidate for prevention of ischemic injury.
Keyword
MeSH Terms
Figure
Reference
-
1. Park JH, Kim YH, Ahn JH, Choi SY, Hong S, Kim SK, Kang IJ, Kim YM, Lee TK, Won MH, Lee CH. Atomoxetine protects against NMDA receptor-mediated hippocampal neuronal death following transient global cerebral ischemia. Curr Neurovasc Res. 2017; 14:158–168.2. Spray S, Edvinsson L. Improved assessment of outcomes following transient global cerebral ischemia in mice. Exp Brain Res. 2016; 234:1925–1934.3. Globus MY, Busto R, Martinez E, Valdés I, Dietrich WD, Ginsberg MD. Comparative effect of transient global ischemia on extracellular levels of glutamate, glycine, and gamma-aminobutyric acid in vulnerable and nonvulnerable brain regions in the rat. J Neurochem. 1991; 57:470–478.4. Lee JC, Park JH, Kim IH, Cho GS, Ahn JH, Tae HJ, Choi SY, Cho JH, Kim DW, Kwon YG, Kang IJ, Won MH, Kim YM. Neuroprotection of ischemic preconditioning is mediated by thioredoxin 2 in the hippocampal CA1 region following a subsequent transient cerebral ischemia. Brain Pathol. 2017; 27:276–291.5. Ye M, Yang W, Ainscough JF, Hu XP, Li X, Sedo A, Zhang XH, Zhang X, Chen Z, Li XM, Beech DJ, Sivaprasadarao A, Luo JH, Jiang LH. TRPM2 channel deficiency prevents delayed cytosolic Zn2+ accumulation and CA1 pyramidal neuronal death after transient global ischemia. Cell Death Dis. 2014; 5:e1541.6. Chun HS, Kim JM, Choi EH, Chang N. Neuroprotective effects of several korean medicinal plants traditionally used for stroke remedy. J Med Food. 2008; 11:246–251.7. Park JH, Cho JH, Kim IH, Ahn JH, Lee JC, Chen BH, Shin BN, Tae HJ, Yoo KY, Hong S, Kang IJ, Won MH, Kim JD. Oenanthe javanica extract protects against experimentally induced ischemic neuronal damage via its antioxidant effects. Chin Med J (Engl). 2015; 128:2932–2937.8. Shi X, Yu W, Yang T, Liu W, Zhao Y, Sun Y, Chai L, Gao Y, Dong B, Zhu L. Panax notoginseng saponins provide neuroprotection by regulating NgR1/RhoA/ROCK2 pathway expression, in vitro and in vivo. J Ethnopharmacol. 2016; 190:301–312.9. Bruch G, Wirth EH. Studies on poplar bud. J Pharm Sci. 1936; 25:672–682.10. Bae KH. The medicinal plants of Korea. Seoul: Kyo-Hak Publishing Co.;1999. p. 98.11. Debbache-Benaida N, Atmani-Kilani D, Schini-Keirth VB, Djebbli N, Atmani D. Pharmacological potential of Populus nigra extract as antioxidant, anti-inflammatory, cardiovascular and hepatoprotective agent. Asian Pac J Trop Biomed. 2013; 3:697–704.12. Zhang X, Thuong PT, Min BS, Ngoc TM, Hung TM, Lee IS, Na M, Seong YH, Song KS, Bae K. Phenolic glycosides with anti-oxidant activity from the stem bark of Populus davidiana. J Nat Prod. 2006; 69:1370–1373.13. Harbilas D, Brault A, Vallerand D, Martineau LC, Saleem A, Arnason JT, Musallam L, Haddad PS. Populus balsamifera L. (Salicaceae) mitigates the development of obesity and improves insulin sensitivity in a diet-induced obese mouse model. J Ethnopharmacol. 2012; 141:1012–1020.14. Karpiak SE, Tagliavia A, Wakade CG. Animal models for the study of drugs in ischemic stroke. Annu Rev Pharmacol Toxicol. 1989; 29:403–414.15. Lee JC, Park JH, Ahn JH, Kim IH, Cho JH, Choi JH, Yoo KY, Lee CH, Hwang IK, Cho JH, Kwon YG, Kim YM, Kang IJ, Won MH. New GABAergic neurogenesis in the hippocampal CA1 region of a gerbil mdel of long-term survival after transient cerebral ischemic injury. Brain Pathol. 2016; 26:581–592.16. Park JH, Shin BN, Chen BH, Kim IH, Ahn JH, Cho JH, Tae HJ, Lee JC, Lee CH, Kim YM, Lee YL, Kim SK, Won MH. Neuroprotection and reduced gliosis by atomoxetine pretreatment in a gerbil model of transient cerebral ischemia. J Neurol Sci. 2015; 359:373–380.17. Gong J, Sun F, Li Y, Zhou X, Duan Z, Duan F, Zhao L, Chen H, Qi S, Shen J. Momordica charantia polysaccharides could protect against cerebral ischemia/reperfusion injury through inhibiting oxidative stress mediated c-Jun N-terminal kinase 3 signaling pathway. Neuropharmacology. 2015; 91:123–134.18. Zhang K, Li YJ, Yang Q, Gerile O, Yang L, Li XB, Guo YY, Zhang N, Feng B, Liu SB, Zhao MG. Neuroprotective effects of oxymatrine against excitotoxicity partially through down-regulation of NR2B-containing NMDA receptors. Phytomedicine. 2013; 20:343–350.19. Golub VM, Brewer J, Wu X, Kuruba R, Short J, Manchi M, Swonke M, Younus I, Reddy DS. Neurostereology protocol for unbiased quantification of neuronal injury and neurodegeneration. Front Aging Neurosci. 2015; 7:196.20. Giulian D, Vaca K. Inflammatory glia mediate delayed neuronal damage after ischemia in the central nervous system. Stroke. 1993; 24:12 Suppl. I84–I90.21. Wang Y, Ge P, Yang L, Wu C, Zha H, Luo T, Zhu Y. Protection of ischemic post conditioning against transient focal ischemia-induced brain damage is associated with inhibition of neuroinflammation via modulation of TLR2 and TLR4 pathways. J Neuroinflammation. 2014; 11:15.22. Pérez-Álvarez MJ, Maza Mdel C, Anton M, Ordoñez L, Wandosell F. Post-ischemic estradiol treatment reduced glial response and triggers distinct cortical and hippocampal signaling in a rat model of cerebral ischemia. J Neuroinflammation. 2012; 9:157.23. Zhang H, Park JH, Maharjan S, Park JA, Choi KS, Park H, Jeong Y, Ahn JH, Kim IH, Lee JC, Cho JH, Lee IK, Lee CH, Hwang IK, Kim YM, Suh YG, Won MH, Kwon YG. Sac-1004, a vascular leakage blocker, reduces cerebral ischemia-reperfusion injury by suppressing blood-brain barrier disruption and inflammation. J Neuroinflammation. 2017; 14:122.24. Ryu JH, Ahn H, Kim JY, Kim YK. Inhibitory activity of plant extracts on nitric oxide synthesis in LPS-activated macrophages. Phytother Res. 2003; 17:485–489.25. Kwon DJ, Bae YS, Ju SM, Youn GS, Choi SY, Park J. Salicortin suppresses lipopolysaccharide-stimulated inflammatory responses via blockade of NF-kappaB and JNK activation in RAW 264.7 macrophages. BMB Rep. 2014; 47:318–323.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Delayed Administration of Green Tea Polyphenol, (-)-pigallocatechin-3-gallate, on the Change of Putrescine Level and Hippocampal Neuronal Cell Damage after Transient Global Ischemia in Gerbil
- Effect of Melatonin on the Changes of Hippocampal Polyamine Content and Neuronal Damage Following Transient Global Ischemia in Mongolian Gerbil: a Study of the Differences of Pre- and Post-ischemic Treatment
- Effect of Ibuprofen on the Changes of Polyamine Level and Neuronal Cell Damage after Transient Global Ischemia in Gerbil
- Effect of Melatonin on Brain Polyamine Contents and Hippocampal Neuronal Damage after Transient Global Ischemia in Mongolian Gerbil
- Effect of Hypothermia on Ischemia-induced Apoptosis: Immunohistochemical Detection of Bax, Caspase-3 and Bcl-2 Proteins