J Periodontal Implant Sci.
2017 Dec;47(6):352-362. 10.5051/jpis.2017.47.6.352.
Periodontal wound healing following reciprocal autologous root transplantation in class III furcation defects
- Affiliations
-
- 1Department of Periodontology, Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan. syoshi@dent.kagoshima-u.ac.jp
- KMID: 2399741
- DOI: http://doi.org/10.5051/jpis.2017.47.6.352
Abstract
- PURPOSE
Furcation involvement in the molars is difficult to treat, and has been recognized as a risk factor for tooth loss. Although periodontal regenerative therapies, including guided tissue regeneration and various types of bone grafts, have been applied to furcation defects, the effects of these treatments are limited, especially in large class III furcation defects. The purpose of this pilot study was to investigate the effect of reciprocal autologous root transplantation on periodontal wound healing and regeneration in class III furcation defects in dogs.
METHODS
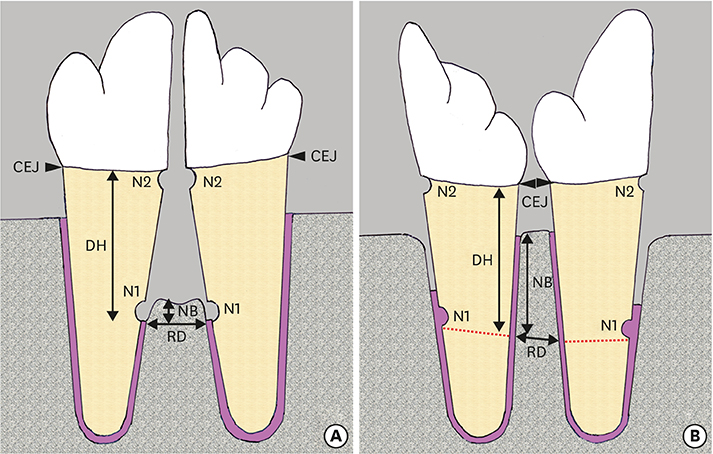
Furcation defects (7 mm wide and 6 mm high) were surgically created after root separation of the unilateral third and fourth premolars in 4 dogs. Eight furcation defects were randomized to receive either reciprocal autologous root transplantation (test) or no further treatment (control). In the test group, the mesial and distal roots were transplanted into the distal and mesial extraction sockets, respectively. The animals were sacrificed 10 weeks after surgery for histologic evaluation.
RESULTS
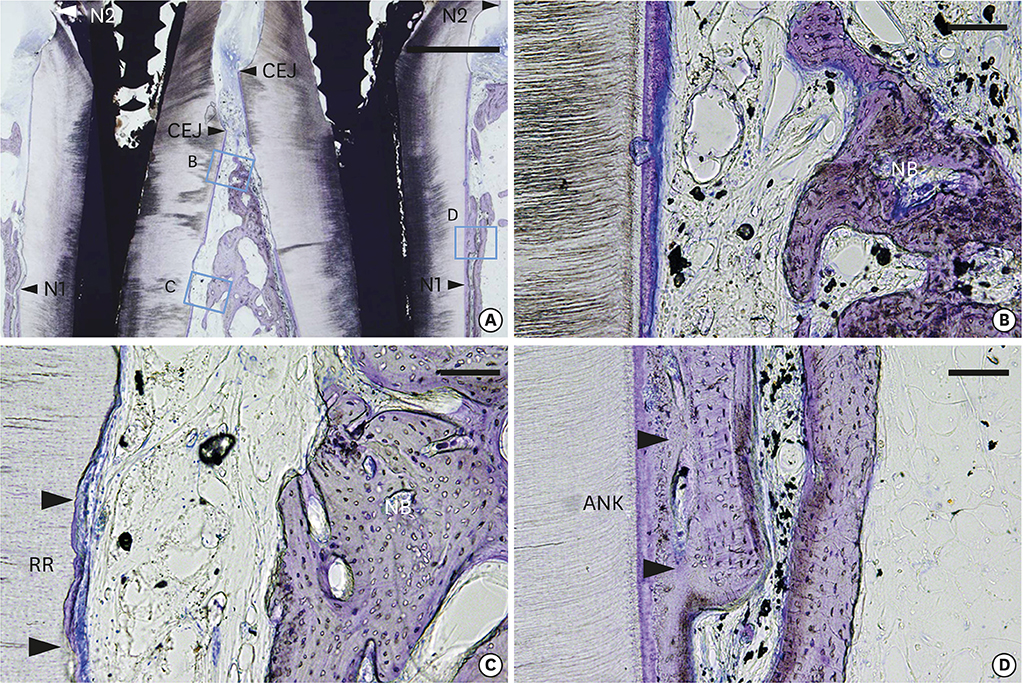
The healing pattern in the control group was characterized by extensive collapse of the flap and limited periodontal regeneration. New bone formation in the test group (3.56±0.57 mm) was significantly greater than in the control group (0.62±0.21 mm). Dense collagen fibers inserting into the residual cementum on the transplanted root surfaces were observed in the test group. Slight ankylosis was observed in 2 of the 4 specimens in the test group on the mesiodistal sides where the root-planed surfaces faced the existing bone. Root resorption (RR) was detected in both the control and test groups.
CONCLUSIONS
Within the limits of this study, it can be concluded that reciprocal autologous root transplantation was effective for bone regeneration in class III furcation defects in dogs. However, further studies are required to standardize the approach in order to prevent unwanted RR prior to clinical application.
MeSH Terms
Figure
Reference
-
1. Al-Shammari KF, Kazor CE, Wang HL. Molar root anatomy and management of furcation defects. J Clin Periodontol. 2001; 28:730–740.
Article2. Nibali L, Zavattini A, Nagata K, Di Iorio A, Lin GH, Needleman I, et al. Tooth loss in molars with and without furcation involvement: a systematic review and meta-analysis. J Clin Periodontol. 2016; 43:156–166.
Article3. Svärdström G, Wennström JL. Furcation topography of the maxillary and mandibular first molars. J Clin Periodontol. 1988; 15:271–275.
Article4. Hou GL, Tsai CC. Cervical enamel projection and intermediate bifurcational ridge correlated with molar furcation involvements. J Periodontol. 1997; 68:687–693.
Article5. Machtei EE, Wasenstein SM, Peretz B, Laufer D. The relationship between cervical enamel projection and class II furcation defects in humans. Quintessence Int. 1997; 28:315–320.6. Pontoriero R, Lindhe J, Nyman S, Karring T, Rosenberg E, Sanavi F. Guided tissue regeneration in the treatment of furcation defects in mandibular molars. A clinical study of degree III involvements. J Clin Periodontol. 1989; 16:170–174.
Article7. Pontoriero R, Nyman S, Ericsson I, Lindhe J. Guided tissue regeneration in surgically-produced furcation defects. An experimental study in the Beagle dog. J Clin Periodontol. 1992; 19:159–163.
Article8. Karring T, Cortellini P. Regenerative therapy: furcation defects. Periodontol 2000. 1999; 19:115–137.
Article9. Jiang J, Wu X, Lin M, Doan N, Xiao Y, Yan F. Application of autologous periosteal cells for the regeneration of class III furcation defects in Beagle dogs. Cytotechnology. 2010; 62:235–243.
Article10. Hale ML. Autogenous transplants. Oral Surg Oral Med Oral Pathol. 1956; 9:76–83.
Article11. Jung RE, Zembic A, Pjetursson BE, Zwahlen M, Thoma DS. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin Oral Implants Res. 2012; 23:Suppl 6. 2–21.12. Mombelli A, Müller N, Cionca N. The epidemiology of peri-implantitis. Clin Oral Implants Res. 2012; 23:Suppl 6. 67–76.
Article13. Andreasen JO, Paulsen HU, Yu Z, Bayer T, Schwartz O. A long-term study of 370 autotransplanted premolars. Part II. Tooth survival and pulp healing subsequent to transplantation. Eur J Orthod. 1990; 12:14–24.
Article14. Lundberg T, Isaksson S. A clinical follow-up study of 278 autotransplanted teeth. Br J Oral Maxillofac Surg. 1996; 34:181–185.
Article15. Josefsson E, Brattström V, Tegsjö U, Valerius-Olsson H. Treatment of lower second premolar agenesis by autotransplantation: four-year evaluation of eighty patients. Acta Odontol Scand. 1999; 57:111–115.
Article16. Tsukiboshi M. Autotransplantation of teeth: requirements for predictable success. Dent Traumatol. 2002; 18:157–180.
Article17. Shimono M, Ishikawa T, Ishikawa H, Matsuzaki H, Hashimoto S, Muramatsu T, et al. Regulatory mechanisms of periodontal regeneration. Microsc Res Tech. 2003; 60:491–502.
Article18. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–155.
Article19. Tamaki Y, Nakahara T, Ishikawa H, Sato S. In vitro analysis of mesenchymal stem cells derived from human teeth and bone marrow. Odontology. 2013; 101:121–132.
Article20. Andreasen JO. Delayed replantation after submucosal storage in order to prevent root resorption after replantation. An experimental study in monkeys. Int J Oral Surg. 1980; 9:394–403.
Article21. Polson AM. Mechanisms of new attachment formation. Endod Dent Traumatol. 1987; 3:45–57.
Article22. Hamamoto Y, Kawasaki N, Jarnbring F, Hammarström L. Effects and distribution of the enamel matrix derivative Emdogain® in the periodontal tissues of rat molars transplanted to the abdominal wall. Dent Traumatol. 2002; 18:12–23.
Article23. Patel S, Dumsha TC, Sydiskis RJ. Determining periodontal ligament (PDL) cell vitality from exarticulated teeth stored in saline or milk using fluorescein diacetate. Int Endod J. 1994; 27:1–5.
Article24. Schwartz O, Andreasen FM, Andreasen JO. Effects of temperature, storage time and media on periodontal and pulpal healing after replantation of incisors in monkeys. Dent Traumatol. 2002; 18:190–195.
Article25. Candeiro GT, Alencar-Júnior EA, Scarparo HC, Furtado-Júnior JH, Gavini G, Caldeira CL. Eight-year follow-up of autogenous tooth transplantation involving multidisciplinary treatment. J Oral Sci. 2015; 57:273–276.
Article26. Lukinmaa PL, Waltimo J. Immunohistochemical localization of types I, V, and VI collagen in human permanent teeth and periodontal ligament. J Dent Res. 1992; 71:391–397.
Article27. Marcopoulou CE, Vavouraki HN, Dereka XE, Vrotsos IA. Proliferative effect of growth factors TGF-beta1, PDGF-BB and rhBMP-2 on human gingival fibroblasts and periodontal ligament cells. J Int Acad Periodontol. 2003; 5:63–70.28. Katayama A, Ota M, Sugito H, Shibukawa Y, Yamada S. Effect of proliferating tissue on transplanted teeth in dogs. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 101:e110–e118.
Article29. Andreasen JO. Periodontal healing after replantation and autotransplantation of incisors in monkeys. Int J Oral Surg. 1981; 10:54–61.
Article30. Iqbal MK, Bamaas N. Effect of enamel matrix derivative (Emdogain®) upon periodontal healing after replantation of permanent incisors in Beagle dogs. Dent Traumatol. 2001; 17:36–45.
Article31. Andreasen JO. A time-related study of periodontal healing and root resorption activity after replantation of mature permanent incisors in monkeys. Swed Dent J. 1980; 4:101–110.32. Oikarinen KS, Stoltze K, Andreasen JO. Influence of conventional forceps extraction and extraction with an extrusion instrument on cementoblast loss and external root resorption of replanted monkey incisors. J Periodontal Res. 1996; 31:337–344.
Article33. Andreasen JO. Interrelation between alveolar bone and periodontal ligament repair after replantation of mature permanent incisors in monkeys. J Periodontal Res. 1981; 16:228–235.
Article34. Suzuki S, Nagano T, Yamakoshi Y, Gomi K, Arai T, Fukae M, et al. Enamel matrix derivative gel stimulates signal transduction of BMP and TGF-β. J Dent Res. 2005; 84:510–514.
Article35. Miron RJ, Sculean A, Cochran DL, Froum S, Zucchelli G, Nemcovsky C, et al. Twenty years of enamel matrix derivative: the past, the present and the future. J Clin Periodontol. 2016; 43:668–683.
Article36. Filippi A, Pohl Y, von Arx T. Treatment of replacement resorption with Emdogain®--a prospective clinical study. Dent Traumatol. 2002; 18:138–143.
Article37. Ninomiya M, Kamata N, Fujimoto R, Ishimoto T, Suryono , Kido J, et al. Application of enamel matrix derivative in autotransplantation of an impacted maxillary premolar: a case report. J Periodontol. 2002; 73:346–351.
Article38. Kim SG, Ryu SI. Enamel matrix derivative for replanted teeth in animal models: a systematic review and meta-analysis. Restor Dent Endod. 2013; 38:194–203.
Article39. Gault PC, Warocquier-Clerout R. Tooth auto-transplantation with double periodontal ligament stimulation to replace periodontally compromised teeth. J Periodontol. 2002; 73:575–583.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Enamel Matrix Derivative on the Healing of Autotransplanted Periodontally Diseased Teeth
- Experimental Study on the Effect of Transforming Growth Factor-beta to Periodontal Regeneration in Class III Furcation Defects
- Experimental study on the effect of direct microcurrent to periodontal regeneration in class III furcation defects
- The comparative study between Dura mater and Guidor(R) in the healing of the classIII furcation defects in dogs
- A Comparative Study of Clinical Healing Aspects in GTR Treatment on Class II Furcation Defects