J Clin Neurol.
2018 Jan;14(1):1-7. 10.3988/jcn.2018.14.1.1.
Roles of the Declive, Folium, and Tuber Cerebellar Vermian Lobules in Sportspeople
- Affiliations
-
- 1Department of Liberal Arts, Kyungil University, Gyeongsan, Korea.
- 2Department of Diagnostic Radiology, Korea University College of Medicine, Seoul, Korea.
- 3Department of Anatomy, Korea University College of Medicine, Seoul, Korea. irhyu@korea.ac.kr
- KMID: 2399592
- DOI: http://doi.org/10.3988/jcn.2018.14.1.1
Abstract
- The cerebellum plays vital roles in balance control and motor learning, including in saccadic adaptation and coordination. It consists of the vermis and two hemispheres and is anatomically separated into ten lobules that are designated as I-X. Although neuroimaging and clinical studies suggest that functions are compartmentalized within the cerebellum, the function of each cerebellar lobule is not fully understood. Electrophysiological and lesion studies in animals as well as neuroimaging and lesion studies in humans have revealed that vermian lobules VI and VII (declive, folium, and tuber) are critical for controlling postural balance, saccadic eye movements, and coordination. In addition, recent structural magnetic resonance imaging studies have revealed that these lobules are larger in elite basketball and short-track speed skaters. Furthermore, in female short-track speed skaters, the volume of this region is significantly correlated with static balance. This article reviews the function of vermian lobules VI and VII, focusing on the control of balance, eye movements, and coordination including coordination between the eyes and hands and bimanual coordination.
Keyword
MeSH Terms
Figure
Reference
-
1. Dow RS, Moruzzi G. The Physiology and Pathology of the Cerebellum. Minneapolis, MN: University of Minnesota Press;1958.2. Holmes G. The cerebellum of man. Brain. 1939; 62:1–30.
Article3. Ito M. The modifiable neuronal network of the cerebellum. Jpn J Physiol. 1984; 34:781–792. PMID: 6099855.
Article4. Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000; 123:1041–1050. PMID: 10775548.5. Riva D, Giorgi C. The cerebellum contributes to higher functions during development: evidence from a series of children surgically treated for posterior fossa tumours. Brain. 2000; 123:1051–1061. PMID: 10775549.
Article6. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998; 121:561–579. PMID: 9577385.
Article7. Jansen J, Brodal A. Experimental studies on the intrinsic fibers of the cerebellum. II. The cortico-nuclear projection. J Comp Neurol. 73:1940; 267–321.
Article8. Larsell O, Jansen J. The comparative anatomy and histology of the cerebellum: the human cerebellum, cerebellar connections, and cerebellar cortex. Minneapolis, MN: The University of Minnesota Press;1972.9. Stoodley CJ, Stein JF. The cerebellum and dyslexia. Cortex. 2011; 47:101–116. PMID: 20060110.
Article10. Dichgans J, Mauritz KH. Patterns and mechanisms of postural instability in patients with cerebellar lesions. Adv Neurol. 1983; 39:633–643. PMID: 6660113.11. Thach WT, Kane SA, Mink JW, Goodkin HP. Cerebellar output: multiple maps and modes of control in movement coordination. In : Llinás R, Sotelo C, editors. The Cerebellum Revisited. New York, NY: Springer-Verlag;1992. p. 283–300.12. Bastian AJ, Mink JW, Kaufman BA, Thach WT. Posterior vermal split syndrome. Ann Neurol. 1998; 44:601–610. PMID: 9778258.
Article13. Growdon JH, Chambers WW, Liu CN. An experimental study of cerebellar dyskinesia in the rhesus monkey. Brain. 1967; 90:603–632. PMID: 4964525.
Article14. Mackel R. The role of the monkey sensory cortex in the recovery from cerebellar injury. Exp Brain Res. 1987; 66:638–652. PMID: 3609206.
Article15. Mason CR, Miller LE, Baker JF, Houk JC. Organization of reaching and grasping movements in the primate cerebellar nuclei as revealed by focal muscimol inactivations. J Neurophysiol. 1998; 79:537–554. PMID: 9463420.
Article16. Leiner HC, Leiner AL, Dow RS. Cognitive and language functions of the human cerebellum. Trends Neurosci. 1993; 16:444–447. PMID: 7507614.
Article17. Middleton FA, Strick PL. Cerebellar output: motor and cognitive channels. Trends Cogn Sci. 1998; 2:348–354. PMID: 21227231.
Article18. Dum RP, Li C, Strick PL. Motor and nonmotor domains in the monkey dentate. Ann N Y Acad Sci. 2002; 978:289–301. PMID: 12582061.
Article19. Ito M. Bases and implications of learning in the cerebellum--adaptive control and internal model mechanism. Prog Brain Res. 2005; 148:95–109. PMID: 15661184.20. Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996; 4:174–198. PMID: 20408197.
Article21. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010; 46:831–844. PMID: 20152963.
Article22. Chheda MG, Sherman JC, Schmahmann JD. Neurologic, psychiatric and cognitive manifestations in cerebellar agenesis. Neurology. 2002; 58:356.23. Tavano A, Grasso R, Gagliardi C, Triulzi F, Bresolin N, Fabbro F, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007; 130:2646–2660. PMID: 17872929.
Article24. MacLullich AM, Edmond CL, Ferguson KJ, Wardlaw JM, Starr JM, Seckl JR, et al. Size of the neocerebellar vermis is associated with cognition in healthy elderly men. Brain Cogn. 2004; 56:344–348. PMID: 15522773.
Article25. Chen SH, Desmond JE. Temporal dynamics of cerebro-cerebellar network recruitment during a cognitive task. Neuropsychologia. 2005; 43:1227–1237. PMID: 15949507.
Article26. Famularo G, Corsi FM, Minisola G, De Simone C, Nicotra GC. Cerebellar tumour presenting with pathological laughter and gelastic syncope. Eur J Neurol. 2007; 14:940–943. PMID: 17662020.
Article27. Richter S, Schoch B, Kaiser O, Groetschel H, Dimitrova A, Hein-Kropp C, et al. Behavioral and affective changes in children and adolescents with chronic cerebellar lesions. Neurosci Lett. 2005; 381:102–107. PMID: 15882798.
Article28. Courchesne E, Yeung-Courchesne R, Press GA, Hesselink JR, Jernigan TL. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988; 318:1349–1354. PMID: 3367935.
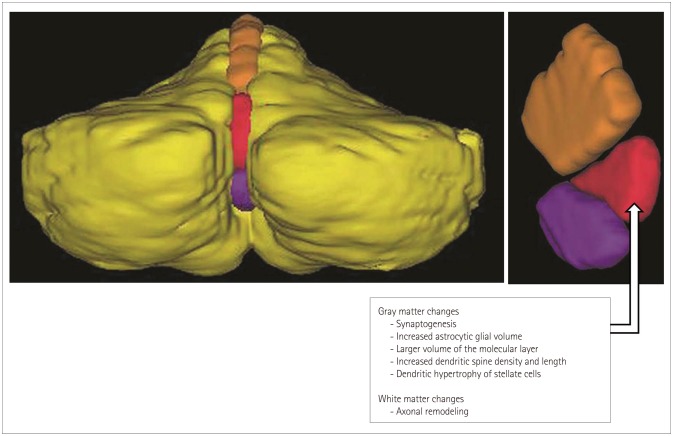
Article29. Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004; 427:311–312. PMID: 14737157.30. Scholz J, Klein MC, Behrens TE, Johansen-Berg H. Training induces changes in white-matter architecture. Nat Neurosci. 2009; 12:1370–1371. PMID: 19820707.
Article31. Park IS, Lee KJ, Han JW, Lee NJ, Lee WT, Park KA, et al. Experience-dependent plasticity of cerebellar vermis in basketball players. Cerebellum. 2009; 8:334–339. PMID: 19259755.
Article32. Park IS, Lee YN, Kwon S, Lee NJ, Rhyu IJ. White matter plasticity in the cerebellum of elite basketball athletes. Anat Cell Biol. 2015; 48:262–267. PMID: 26770877.
Article33. Park IS, Lee NJ, Kim TY, Park JH, Won YM, Jung YJ, et al. Volumetric analysis of cerebellum in short-track speed skating players. Cerebellum. 2012; 11:925–930. PMID: 22351379.
Article34. Park IS, Yoon JH, Kim N, Rhyu IJ. Regional cerebellar volume reflects static balance in elite female short-track speed skaters. Int J Sports Med. 2013; 34:465–470. PMID: 23143696.
Article35. Wei G, Luo J, Li Y. Brain structure in diving players on MR imaging studied with voxel-based morphometry. Prog Nat Sci. 2009; 19:1397–1402.
Article36. Hüfner K, Binetti C, Hamilton DA, Stephan T, Flanagin VL, Linn J, et al. Structural and functional plasticity of the hippocampal formation in professional dancers and slackliners. Hippocampus. 2011; 21:855–865. PMID: 20572197.
Article37. Di X, Zhu S, Jin H, Wang P, Ye Z, Zhou K, et al. Altered resting brain function and structure in professional badminton players. Brain Connect. 2012; 2:225–233. PMID: 22840241.
Article38. Di Paola M, Caltagirone C, Petrosini L. Prolonged rock climbing activity induces structural changes in cerebellum and parietal lobe. Hum Brain Mapp. 2013; 34:2707–2714. PMID: 22522914.
Article39. Hänggi J, Langer N, Lutz K, Birrer K, Mérillat S, Jäncke L. Structural brain correlates associated with professional handball playing. PLoS One. 2015; 10:e0124222. PMID: 25915906.
Article40. Botterell EH, Fulton JG. Functional localization in the cerebellum of primates II. Lesions of midline structures (vermis) and deep nuclei. J Comp Neurol. 1938; 69:47–62.
Article41. Chambers WW, Sprague JM. Functional localization in the cerebellum. I. Organization in longitudinal cortico-nuclear zones and their contribution to the control of posture, both extrapyramidal and pyramidal. J Comp Neurol. 1955; 103:105–129. PMID: 13263445.
Article42. Ilg W, Giese MA, Gizewski ER, Schoch B, Timmann D. The influence of focal cerebellar lesions on the control and adaptation of gait. Brain. 2008; 131:2913–2927. PMID: 18835866.
Article43. Konczak J, Schoch B, Dimitrova A, Gizewski E, Timmann D. Functional recovery of children and adolescents after cerebellar tumour resection. Brain. 2005; 128:1428–1441. PMID: 15659424.
Article44. Schoch B, Hogan A, Gizewski ER, Timmann D, Konczak J. Balance control in sitting and standing in children and young adults with benign cerebellar tumors. Cerebellum. 2010; 9:324–335. PMID: 20352395.
Article45. Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992; 15:403–442. PMID: 1575449.
Article46. Kotchabhakdi N, Walberg F. Primary vestibular afferent projections to the cerebellum as demonstrated by retrograde axonal transport of horseradish peroxidase. Brain Res. 1978; 142:142–146. PMID: 75045.
Article47. Clendenin M, Ekerot CF, Oscarsson O, Rosén I. Functional organization of two spinocerebellar paths relayed through the lateral reticular nucleus in the cat. Brain Res. 1974; 69:140–143. PMID: 4817908.
Article48. Matsushita M, Okado N. Spinocerebellar projections to lobules I and II of the anterior lobe in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol. 1981; 197:411–424. PMID: 6163798.
Article49. Morton SM, Bastian AJ. Cerebellar control of balance and locomotion. Neuroscientist. 2004; 10:247–259. PMID: 15155063.
Article50. Asanuma C, Thach WT, Jones EG. Brainstem and spinal projections of the deep cerebellar nuclei in the monkey, with observations on the brainstem projections of the dorsal column nuclei. Brain Res. 1983; 286:299–322. PMID: 6189563.
Article51. Sprague JM, Chambers WW. Regulation of posture in intact and decerebrate cat. I. Cerebellum, reticular formation, vestibular nuclei. J Neurophysiol. 1953; 16:451–463. PMID: 13097194.
Article52. Botterell EH, Fulton JF. Functional localization in the cerebellum of primates III. Lesions of hemispheres (neocerebellum). J Comp Neurol. 1938; 69:63–87.
Article53. Glickstein M. How are visual areas of the brain connected to motor areas for the sensory guidance of movement? Trends Neurosci. 2000; 23:613–617. PMID: 11137151.
Article54. Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001; 21:700–712. PMID: 11160449.
Article55. Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003; 89:634–639. PMID: 12522208.
Article56. Coffman KA, Dum RP, Strick PL. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc Natl Acad Sci U S A. 2011; 108:16068–16073. PMID: 21911381.
Article57. Schoch B, Konczak J, Dimitrova A, Gizewski ER, Wieland R, Timmann D. Impact of surgery and adjuvant therapy on balance function in children and adolescents with cerebellar tumors. Neuropediatrics. 2006; 37:350–358. PMID: 17357037.
Article58. Schoch B, Dimitrova A, Gizewski ER, Timmann D. Functional localization in the human cerebellum based on voxelwise statistical analysis: a study of 90 patients. Neuroimage. 2006; 30:36–51. PMID: 16253526.
Article59. Brandauer B, Hermsdörfer J, Beck A, Aurich V, Gizewski ER, Marquardt C, et al. Impairments of prehension kinematics and grasping forces in patients with cerebellar degeneration and the relationship to cerebellar atrophy. Clin Neurophysiol. 2008; 119:2528–2537. PMID: 18835217.
Article60. Siebold C, Kleine JF, Glonti L, Tchelidze T, Büttner U. Fastigial nucleus activity during different frequencies and orientations of vertical vestibular stimulation in the monkey. J Neurophysiol. 1999; 82:34–41. PMID: 10400932.
Article61. Thach WT. Discharge of cerebellar neurons related to two maintained postures and two prompt movements. I. Nuclear cell output. J Neurophysiol. 1970; 33:527–536. PMID: 4988214.
Article62. Imperato A, Nicoletti F, Diana M, Scapagnini U, Di Chiara G. Fastigial influences on postural tonus as studied by kainate lesions and by local infusion of GABAergic drugs in the rat. Brain Res. 1984; 295:51–63. PMID: 6370373.
Article63. Mauritz KH, Dichgans J, Hufschmidt A. Quantitative analysis of stance in late cortical cerebellar atrophy of the anterior lobe and other forms of cerebellar ataxia. Brain. 1979; 102:461–482. PMID: 315255.
Article64. Diener HC, Dichgans J, Bacher M, Guschlbauer B. Characteristic alterations of long-loop “reflexes” in patients with Friedreich's disease and late atrophy of the cerebellar anterior lobe. J Neurol Neurosurg Psychiatry. 1984; 47:679–685. PMID: 6747644.
Article65. Baloh RW, Yee RD, Honrubia V. Late cortical cerebellar atrophy. Clinical and oculographic features. Brain. 1986; 109:159–180. PMID: 3484651.66. Sullivan EV, Rose J, Pfefferbaum A. Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cereb Cortex. 2006; 16:1077–1086. PMID: 16221930.
Article67. Bloedel JR, Courville J. Cerebellar afferent systems. In : Brooks VB, editor. Handbook of Physiology, the nervous system. Bethesda: American Physiological Society;1981. p. 735–829.68. Brodal A. Afferent cerebellar connections. In : Jansen J, Brodal A, editors. Aspects of cerebellar anatomy. Oslo: Johan Grundt Tanum Forlag;1954. p. 82–177.69. Ye BS, Kim YD, Nam HS, Lee HS, Nam CM, Heo JH. Clinical manifestations of cerebellar infarction according to specific lobular involvement. Cerebellum. 2010; 9:571–579. PMID: 20711853.
Article70. Mitchell J. Postural reactions of the lower limb to centre-of-gravity displacements in one-foot stance. Physiotherapy. 1971; 57:562–570. PMID: 5145738.71. Richardson JK, Ashton-Miller JA, Lee SG, Jacobs K. Moderate peripheral neuropathy impairs weight transfer and unipedal balance in the elderly. Arch Phys Med Rehabil. 1996; 77:1152–1156. PMID: 8931527.
Article72. Doya K. Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol. 2000; 10:732–739. PMID: 11240282.
Article73. Hopp JJ, Fuchs AF. The characteristics and neuronal substrate of saccadic eye movement plasticity. Prog Neurobiol. 2004; 72:27–53. PMID: 15019175.
Article74. Kojima Y, Soetedjo R, Fuchs AF. Changes in simple spike activity of some Purkinje cells in the oculomotor vermis during saccade adaptation are appropriate to participate in motor learning. J Neurosci. 2010; 30:3715–3727. PMID: 20220005.
Article75. Noda H, Fujikado T. Involvement of Purkinje cells in evoking saccadic eye movements by microstimulation of the posterior cerebellar vermis of monkeys. J Neurophysiol. 1987; 57:1247–1261. PMID: 3585467.
Article76. Noda H, Fujikado T. Topography of the oculomotor area of the cerebellar vermis in macaques as determined by microstimulation. J Neurophysiol. 1987; 58:359–378. PMID: 3655873.
Article77. Noda H, Sugita S, Ikeda Y. Afferent and efferent connections of the oculomotor region of the fastigial nucleus in the macaque monkey. J Comp Neurol. 1990; 302:330–348. PMID: 1705268.
Article78. Helmchen C, Büttner U. Saccade-related Purkinje cell activity in the oculomotor vermis during spontaneous eye movements in light and darkness. Exp Brain Res. 1995; 103:198–208. PMID: 7789427.
Article79. Ron S, Robinson DA. Eye movements evoked by cerebellar stimulation in the alert monkey. J Neurophysiol. 1973; 36:1004–1022. PMID: 4202613.
Article80. Fujikado T, Noda H. Saccadic eye movements evoked by microstimulation of lobule VII of the cerebellar vermis of macaque monkeys. J Physiol. 1987; 394:573–594. PMID: 3443975.
Article81. Mottolese C, Richard N, Harquel S, Szathmari A, Sirigu A, Desmurget M. Mapping motor representations in the human cerebellum. Brain. 2013; 136:330–342. PMID: 22945964.
Article82. Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Thier P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci. 1999; 19:10931–10939. PMID: 10594074.
Article83. Takagi M, Zee DS, Tamargo RJ. Effects of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol. 1998; 80:1911–1931. PMID: 9772249.
Article84. Hayakawa Y, Nakajima T, Takagi M, Fukuhara N, Abe H. Human cerebellar activation in relation to saccadic eye movements: a functional magnetic resonance imaging study. Ophthalmologica. 2002; 216:399–405. PMID: 12566881.
Article85. Nitschke MF, Arp T, Stavrou G, Erdmann C, Heide W. The cerebellum in the cerebro-cerebellar network for the control of eye and hand movements--an fMRI study. Prog Brain Res. 2005; 148:151–164. PMID: 15661188.86. Stephan T, Mascolo A, Yousry TA, Bense S, Brandt T, Dieterich M. Changes in cerebellar activation pattern during two successive sequences of saccades. Hum Brain Mapp. 2002; 16:63–70. PMID: 11954056.
Article87. Desmurget M, Pélisson D, Urquizar C, Prablanc C, Alexander GE, Grafton ST. Functional anatomy of saccadic adaptation in humans. Nat Neurosci. 1998; 1:524–528. PMID: 10196552.
Article88. Desmurget M, Pélisson D, Grethe JS, Alexander GE, Urquizar C, Prablanc C, et al. Functional adaptation of reactive saccades in humans: a PET study. Exp Brain Res. 2000; 132:243–259. PMID: 10853949.
Article89. Jenkinson N, Miall RC. Disruption of saccadic adaptation with repetitive transcranial magnetic stimulation of the posterior cerebellum in humans. Cerebellum. 2010; 9:548–555. PMID: 20665254.
Article90. Golla H, Tziridis K, Haarmeier T, Catz N, Barash S, Thier P. Reduced saccadic resilience and impaired saccadic adaptation due to cerebellar disease. Eur J Neurosci. 2008; 27:132–144. PMID: 18184318.
Article91. Straube A, Deubel H, Ditterich J, Eggert T. Cerebellar lesions impair rapid saccade amplitude adaptation. Neurology. 2001; 57:2105–2108. PMID: 11739834.
Article92. Choi KD, Kim HJ, Cho BM, Kim JS. Saccadic adaptation in lateral medullary and cerebellar infarction. Exp Brain Res. 2008; 188:475–482. PMID: 18421449.
Article93. Miall RC, Jenkinson EW. Functional imaging of changes in cerebellar activity related to learning during a novel eye-hand tracking task. Exp Brain Res. 2005; 166:170–183. PMID: 16082535.
Article94. Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Changes in brain activation during the acquisition of a new bimanual coodination task. Neuropsychologia. 2004; 42:855–867. PMID: 14998701.95. Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP. Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage. 2004; 21:1416–1427. PMID: 15050567.
Article96. Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990; 87:5568–5572. PMID: 1695380.
Article97. Anderson BJ, Alcantara AA, Greenough WT. Motor-skill learning: changes in synaptic organization of the rat cerebellar cortex. Neurobiol Learn Mem. 1996; 66:221–229. PMID: 8946414.
Article98. Kleim JA, Swain RA, Armstrong KA, Napper RM, Jones TA, Greenough WT. Selective synaptic plasticity within the cerebellar cortex following complex motor skill learning. Neurobiol Learn Mem. 1998; 69:274–289. PMID: 9707490.
Article99. Federmeier KD, Kleim JA, Greenough WT. Learning-induced multiple synapse formation in rat cerebellar cortex. Neurosci Lett. 2002; 332:180–184. PMID: 12399010.
Article100. Kim HT, Kim IH, Lee KJ, Lee JR, Park SK, Chun YH, et al. Specific plasticity of parallel fiber/Purkinje cell spine synapses by motor skill learning. Neuroreport. 2002; 13:1607–1610. PMID: 12352611.
Article101. Anderson BJ, Li X, Alcantara AA, Isaacs KR, Black JE, Greenough WT. Glial hypertrophy is associated with synaptogenesis following motor-skill learning, but not with angiogenesis following exercise. Glia. 1994; 11:73–80. PMID: 7520887.
Article102. Kleim JA, Markham JA, Vij K, Freese JL, Ballard DH, Greenough WT. Motor learning induces astrocytic hypertrophy in the cerebellar cortex. Behav Brain Res. 2007; 178:244–249. PMID: 17257689.
Article103. Lee KJ, Jung JG, Arii T, Imoto K, Rhyu IJ. Morphological changes in dendritic spines of Purkinje cells associated with motor learning. Neurobiol Learn Mem. 2007; 88:445–450. PMID: 17720555.
Article