Lab Med Online.
2018 Jan;8(1):19-23. 10.3343/lmo.2018.8.1.19.
Internal Quality Assurance Status of Stool Examination as Assessed by a Questionnaire in Korean Clinical Laboratories
- Affiliations
-
- 1Department of Laboratory Medicine, Chonnam National University Medical School, Gwangju, Korea. dana_clinic@naver.com
- KMID: 2398415
- DOI: http://doi.org/10.3343/lmo.2018.8.1.19
Abstract
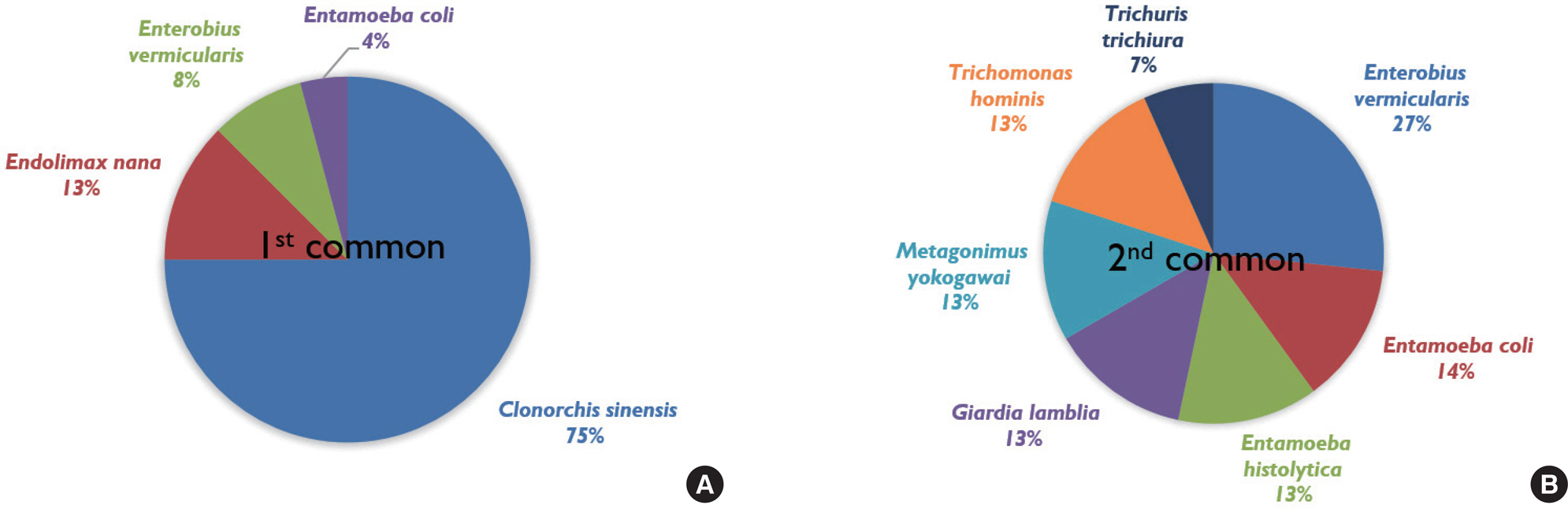
- This study aimed to survey the status of quality control (QC) assurance for stool examinations at clinical laboratories in Korea. We sent a questionnaire related to QC practices in stool examination by electronic mail to Korean clinical laboratories that performed stool examination. Overall, 20 of the 39 laboratories (51.3%) reported performing stool concentration methods, and 28 (71.8%) examined the slides using only saline. A large proportion (74.4%) of respondents did not check the internal QC because of the restriction of appropriate control materials. Only four laboratories (10.3%) checked the reactivity of the dye solution routinely. For appropriate external QC systems, QC slides (43.6%) were preferred, followed by QC materials (30.8%), virtual slides (17.9%), and a combination of the above options (7.7%). The most commonly observed parasites in stool samples at the clinical laboratories were Clonorchis sinensis (75%), followed by Endolimax nana, Enterobius vermicularis, and Entamoeba coli. The present study describes the difficulties in internal QC assessment due to the absence of standardized QC materials and systems. We hope the findings of this report will provide a foundation for a QC assessment program for stool examinations in the near future.
Keyword
MeSH Terms
Figure
Reference
-
1.World Health Organization. The global burden of disease: 2004 update. Geneva, Switzerland: World Health Organization. 2008.2.Clinical and Laboratory Standards Institute. Procedures for the recovery and identifcation of parasites from the intestinal tract; approved guideline. 2nd ed.CLSI document M28-A2. Wayne, PA: Clinical and Laboratory Standards Institute;2005.3.Dovgalev AS., Astanina SY., Malakhov VN., Serdyuk AP., Imamkuliev KD., Gorbunova YP, et al. External quality assessment for the laboratory identifcation of the pathogens of parasitic diseases as an element for improving the postgraduate training of specialists. Med Parazitol (Mosk). 2016. 2:41–4.4.Chang J., Kim MN., Kim EC., Shin JH., Lee NY., Kim S, et al. Annual report on the external quality assessment scheme for clinical microbiology in Korea (2015). J Lab Med Qual Assur. 2016. 38:169–93.
Article5.Manser MM., Saez ACS., Chiodini PL. Faecal parasitology: Concentration methodology needs to be better standardized. PLoS Negl Trop Dis. 2016. 10:e0004579.6.Verweij JJ., Blangé RA., Templeton K., Schinkel J., Brienen EA., van Rooyen MA, et al. Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J Clin Microbiol. 2004. 42:1220–3.7.Stark D., Al-Qassab SE., Barratt JL., Stanley K., Roberts T., Marriott D, et al. Evaluation of multiplex tandem real-time PCR for detection of Cryp-tosporidium spp., Dientamoeba fragilis, Entamoeba histolytica, and Giardia intestinalis in clinical stool samples. J Clin Microbiol. 2011. 49:257–62.
Article8.Won EJ., Kim SH., Kee SJ., Shin JH., Suh SP., Chai JY, et al. Multiplex realtime PCR assay targeting eight parasites customized to the Korean population: potential use for detection in diarrheal stool samples from gastroenteritis patients. PLos One. 2016. 11:e0166957.
Article9.Gardner BB., Del Junco DJ., Fenn J., Hengesbaugh JH. Comparision of direct wet mount and trichrome staining techniques for detecting Entamoeba species trophozoites in stools. J Clin Microbiol. 1980. 12:656–8.10.Libman MD., Gyorkos TW., Kokoskin E., Maclean JD. Detection of pathogenic protozoa in the diagnostic laboratory: result reproducibility, specimen pooling, and competency assessment. J Clin Microbiol. 2008. 46:2200–5.
Article11.Linder E., Lundin M., Thors C., Lebbad M., Winiecka-Krusnell J., Helin H, et al. Web-based virtual microscopy for parasitology: a novel tool for education and quality assurance. PLoS Negl Trop Dis. 2008. 2:e315.
Article12.Weitzel T., Dittrich S., Möhl I., Adusu E., Jelinek T. Evaluation of seven commercial antigen detection tests for Giardia and Cryptosporidium in stool samples. Clin Microbiol Infect. 2006. 12:656–9.
Article13.Jahan N., Khatoon R., Ahmad S. A comparison of microscopy and enzyme linked immunosorbent assay for diagnosis of Giardia lamblia in human faecal specimens. J Clin Diagn Res. 2014. 8:DC04–6.14.Korea Centers for Disease Control and Prevention, Korea Association of Health Promotion. National survey of the prevalence of intestinal parasitic infections in Korea, 2012. The 8th Report. Osong Chungcheon-gbuk-do, Korea, 2013. of Health Promotion. National survey of the prevalence of intestinal.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Survey and Solutions for the Current Status of Quality Control in Small Hospital Laboratories
- Survey of Eight Hormone Tests Used by Clinical Laboratories in Korea
- Survey of Fungal Cultures and the Identification Tests Used by Diagnostic Laboratories in Korea
- Annual Report on External Quality Assessment in Diagnostic Genetics in Korea (1997)
- The Current Status of HIV Serologic Testing in Korean Clinical Laboratories during the Year 2007