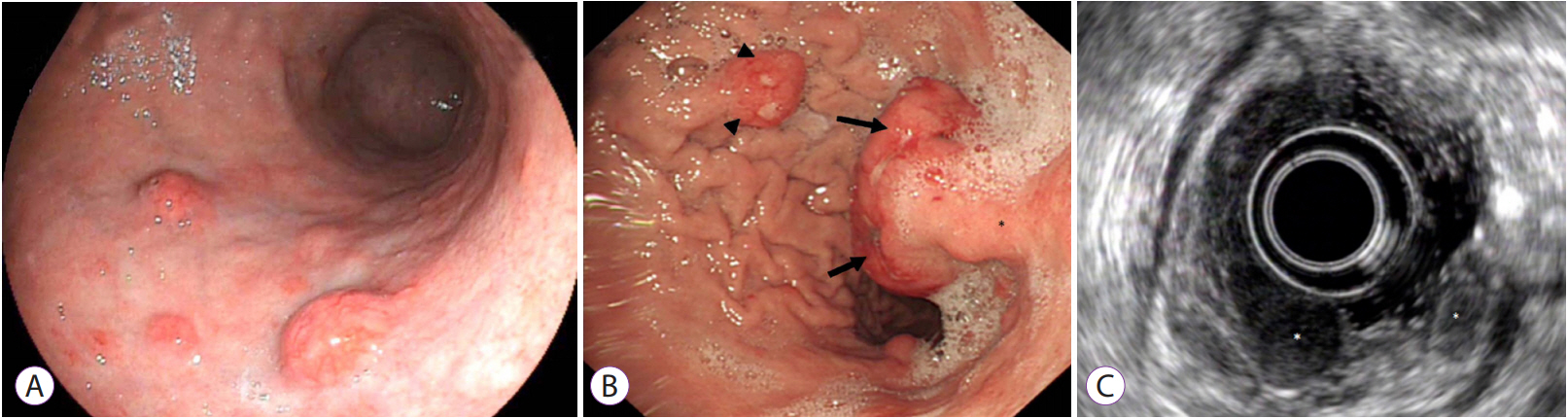
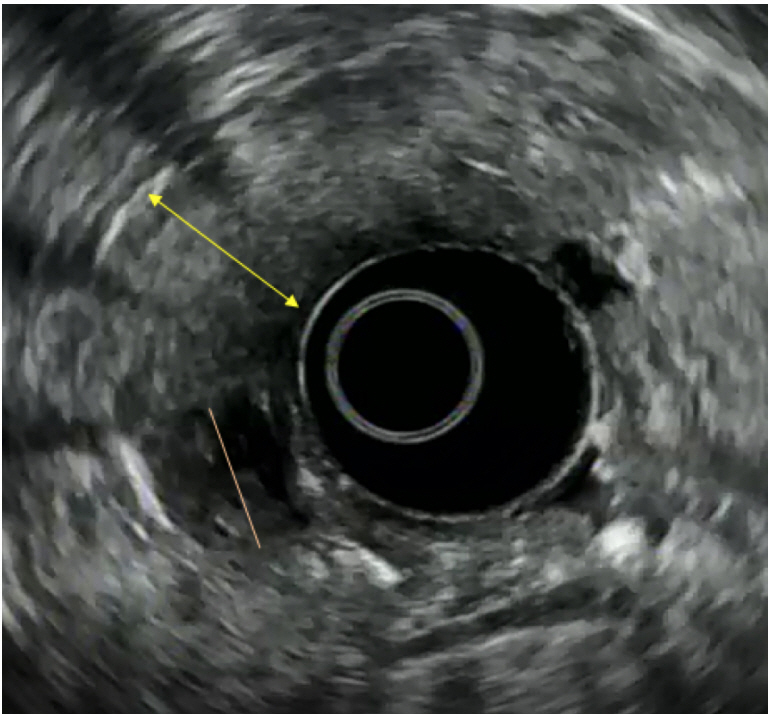
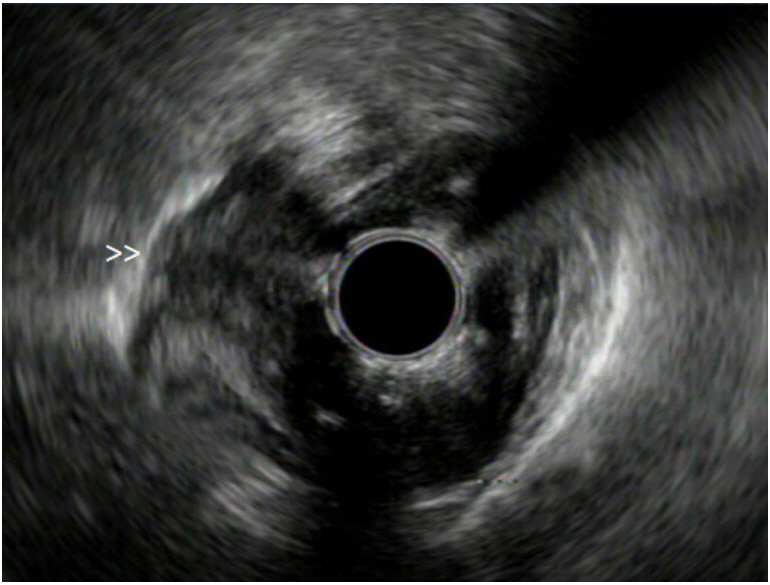
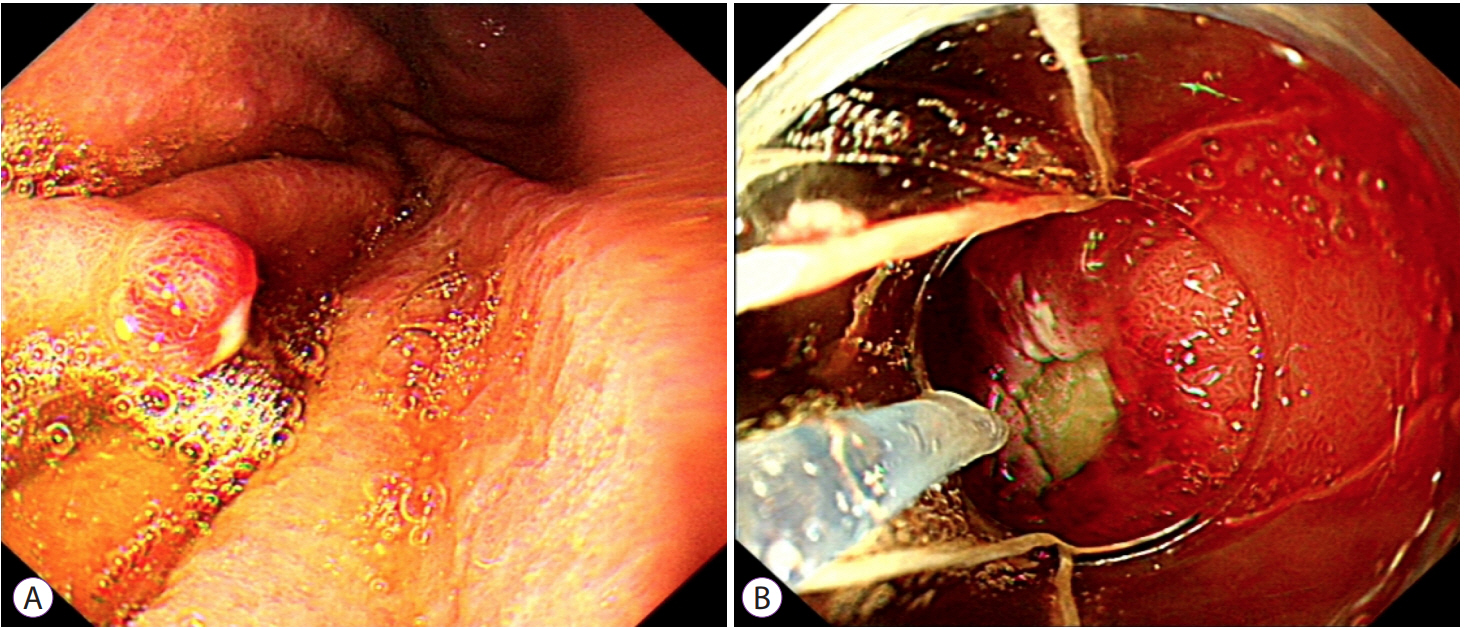
Clin Endosc.
2017 Nov;50(6):520-529. 10.5946/ce.2017.181.
Diagnosis and Management of Upper Gastrointestinal Neuroendocrine Tumors
- Affiliations
-
- 1Department of Gastroenterology, St. Vincent's University Hospital, University College Dublin, Dublin, Ireland. otooled1@tcd.ie
- 2Department of Clinical Medicine, Trinity College Dublin, The University of Dublin, Dublin, Ireland.
- KMID: 2398302
- DOI: http://doi.org/10.5946/ce.2017.181
Abstract
- Upper gastrointestinal neuroendocrine tumors (NETs) are rare tumors which are increasingly recognised by practising endoscopists. After confirmation by endoscopic biopsies of these focal lesions, many questions may arise. As NETs are less frequently encountered compared to other malignancies or gastrointestinal pathology, many endoscopists may not fully understand the natural history, diagnosis and management of these tumors. In this review, we aim to update the practising endoscopist on the key clinical features and management of patients with upper gastrointestinal NET.
Keyword
Figure
Reference
-
1. Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008; 26:3063–3072.
Article2. Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003; 97:934–959.
Article3. Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol. 2004; 99:23–32.
Article4. Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017; 3:1335–1342.
Article5. Huang Q, Wu H, Nie L, et al. Primary high-grade neuroendocrine carcinoma of the esophagus: a clinicopathologic and immunohistochemical study of 42 resection cases. Am J Surg Pathol. 2013; 37:467–483.6. Lee CG, Lim YJ, Park SJ, et al. The clinical features and treatment modality of esophageal neuroendocrine tumors: a multicenter study in Korea. BMC Cancer. 2014; 14:569.
Article7. Babu Kanakasetty G, Dasappa L, Lakshmaiah KC, et al. Clinicopathological profile of pure neuroendocrine neoplasms of the esophagus: a South Indian center experience. J Oncol. 2016; 2016:2402417.
Article8. Lv J, Liang J, Wang J, et al. Primary small cell carcinoma of the esophagus. J Thorac Oncol. 2008; 3:1460–1465.
Article9. Bennouna J, Bardet E, Deguiral P, Douillard JY. Small cell carcinoma of the esophagus: analysis of 10 cases and review of the published data. Am J Clin Oncol. 2000; 23:455–459.10. Ku GY, Minsky BD, Rusch VW, Bains M, Kelsen DP, Ilson DH. Smallcell carcinoma of the esophagus and gastroesophageal junction: review of the Memorial Sloan-Kettering experience. Ann Oncol. 2008; 19:533–537.
Article11. Kukar M, Groman A, Malhotra U, et al. Small cell carcinoma of the esophagus: a SEER database analysis. Ann Surg Oncol. 2013; 20:4239–4244.
Article12. Vos B, Rozema T, Miller RC, et al. Small cell carcinoma of the esophagus: a multicentre rare cancer network study. Dis Esophagus. 2011; 24:258–264.
Article13. Egashira A, Morita M, Kumagai R, et al. Neuroendocrine carcinoma of the esophagus: clinicopathological and immunohistochemical features of 14 cases. PLoS One. 2017; 12:e0173501.
Article14. Deng HY, Ni PZ, Wang YC, Wang WP, Chen LQ. Neuroendocrine carcinoma of the esophagus: clinical characteristics and prognostic evaluation of 49 cases with surgical resection. J Thorac Dis. 2016; 8:1250–1256.
Article15. Wong AT, Shao M, Rineer J, Osborn V, Schwartz D, Schreiber D. Treatment and survival outcomes of small cell carcinoma of the esophagus: an analysis of the national cancer data base. Dis Esophagus. 2017; 30:1–5.
Article16. Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993; 104:994–1006.
Article17. O’Toole D, Delle Fave G, Jensen RT. Gastric and duodenal neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012; 26:719–735.18. Delle Fave G, Kwekkeboom DJ, Van Cutsem E, et al. ENETS consensus guidelines for the management of patients with gastroduodenal neoplasms. Neuroendocrinology. 2012; 95:74–87.
Article19. Delle Fave G, O’Toole D, Sundin A, et al. ENETS consensus guidelines update for gastroduodenal neuroendocrine neoplasms. Neuroendocrinology. 2016; 103:119–124.
Article20. Niederle MB, Hackl M, Kaserer K, Niederle B. Gastroenteropancreatic neuroendocrine tumours: the current incidence and staging based on the WHO and European neuroendocrine tumour society classification: an analysis based on prospectively collected parameters. Endocr Relat Cancer. 2010; 17:909–918.
Article21. Tsai HJ, Wu CC, Tsai CR, Lin SF, Chen LT, Chang JS. The epidemiology of neuroendocrine tumors in Taiwan: a nation-wide cancer registry-based study. PLoS One. 2013; 8:e62487.
Article22. Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015; 121:589–597.
Article23. Pavel M, de Herder WW. ENETS consensus guidelines for the standard of care in neuroendocrine tumors. Neuroendocrinology. 2017; 105:193–195.24. Sundin A, Arnold R, Baudin E, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: radiological, nuclear medicine & hybrid imaging. Neuroendocrinology. 2017; 105:212–244.25. Campana D, Ravizza D, Ferolla P, et al. Risk factors of type 1 gastric neuroendocrine neoplasia in patients with chronic atrophic gastritis. A retrospective, multicentre study. Endocrine. 2017; 56:633–638.
Article26. Roy PK, Venzon DJ, Shojamanesh H, et al. Zollinger-Ellison syndrome. Clinical presentation in 261 patients. Medicine (Baltimore). 2000; 79:379–411.
Article27. O’Toole D, Palazzo L. Endoscopy and endoscopic ultrasound in assessing and managing neuroendocrine neoplasms. Front Horm Res. 2015; 44:88–103.28. Rindi G, Arnold R, Bosman FT. Nomenclature and classification of neuroendocrine neoplasms of the digestive system. In : Bosman FT, Carneiro F, Hruban RH, editors. WHO classification of tumours of the digestive system 4th ed. Lyon: International Agency for Research on Cancer (IARC);2010. p. 13.29. Schindl M, Kaserer K, Niederle B. Treatment of gastric neuroendocrine tumors: the necessity of a type-adapted treatment. Arch Surg. 2001; 136:49–54.30. Borch K, Ahrén B, Ahlman H, Falkmer S, Granérus G, Grimelius L. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Ann Surg. 2005; 242:64–73.31. Park YH, Kim N. Review of atrophic gastritis and intestinal metaplasia as a premalignant lesion of gastric cancer. J Cancer Prev. 2015; 20:25–40.
Article32. O’Connor A, McNamara D, O’Moráin CA. Surveillance of gastric intestinal metaplasia for the prevention of gastric cancer. Cochrane Database Syst Rev. 2013; CD009322.
Article33. Kunz PL, Reidy-Lagunes D, Anthony LB, et al. Consensus guidelines for the management and treatment of neuroendocrine tumors. Pancreas. 2013; 42:557–577.
Article34. Merola E, Sbrozzi-Vanni A, Panzuto F, et al. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology. 2012; 95:207–213.
Article35. Li QL, Zhang YQ, Chen WF, et al. Endoscopic submucosal dissection for foregut neuroendocrine tumors: an initial study. World J Gastroenterol. 2012; 18:5799–5806.
Article36. Sato Y, Takeuchi M, Hashimoto S, et al. Usefulness of endoscopic submucosal dissection for type I gastric carcinoid tumors compared with endoscopic mucosal resection. Hepatogastroenterology. 2013; 60:1524–1529.37. Kim HH, Kim GH, Kim JH, Choi MG, Song GA, Kim SE. The efficacy of endoscopic submucosal dissection of type I gastric carcinoid tumors compared with conventional endoscopic mucosal resection. Gastroenterol Res Pract. 2014; 2014:253860.
Article38. Campana D, Nori F, Pezzilli R, et al. Gastric endocrine tumors type I: treatment with long-acting somatostatin analogs. Endocr Relat Cancer. 2008; 15:337–342.
Article39. Jianu CS, Fossmark R, Syversen U, Hauso Ø, Fykse V, Waldum HL. Fiveyear follow-up of patients treated for 1 year with octreotide long-acting release for enterochromaffin-like cell carcinoids. Scand J Gastroenterol. 2011; 46:456–463.
Article40. Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the WHO classification. Ann N Y Acad Sci. 2004; 1014:13–27.41. Okubo Y, Yokose T, Motohashi O, et al. Duodenal rare neuroendocrine tumor: clinicopathological characteristics of patients with gangliocytic paraganglioma. Gastroenterol Res Pract. 2016; 2016:5257312.
Article42. Hoffmann KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: classification, functional syndromes, diagnosis and medical treatment. Best Pract Res Clin Gastroenterol. 2005; 19:675–697.
Article43. Stamm B, Hedinger CE, Saremaslani P. Duodenal and ampullary carcinoid tumors. A report of 12 cases with pathological characteristics, polypeptide content and relation to the MEN I syndrome and von Recklinghausen’s disease (neurofibromatosis). Virchows Arch A Pathol Anat Histopathol. 1986; 408:475–489.44. Benya RV, Metz DC, Venzon DJ, et al. Zollinger-Ellison syndrome can be the initial endocrine manifestation in patients with multiple endocrine neoplasia-type I. Am J Med. 1994; 97:436–444.
Article45. Gibril F, Schumann M, Pace A, Jensen RT. Multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome: a prospective study of 107 cases and comparison with 1009 cases from the literature. Medicine (Baltimore). 2004; 83:43–83.46. Yu F, Venzon DJ, Serrano J, et al. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol. 1999; 17:615–630.
Article47. Weber HC, Venzon DJ, Lin JT, et al. Determinants of metastatic rate and survival in patients with Zollinger-Ellison syndrome: a prospective long-term study. Gastroenterology. 1995; 108:1637–1649.
Article48. Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab. 2012; 97:2990–3011.
Article49. Dalenbäck J, Havel G. Local endoscopic removal of duodenal carcinoid tumors. Endoscopy. 2004; 36:651–655.
Article50. Kim GH, Kim JI, Jeon SW, et al. Endoscopic resection for duodenal carcinoid tumors: a multicenter, retrospective study. J Gastroenterol Hepatol. 2014; 29:318–324.
Article51. Matsumoto S, Miyatani H, Yoshida Y, Nokubi M. Duodenal carcinoid tumors: 5 cases treated by endoscopic submucosal dissection. Gastrointest Endosc. 2011; 74:1152–1156.
Article52. Suzuki S, Ishii N, Uemura M, et al. Endoscopic submucosal dissection (ESD) for gastrointestinal carcinoid tumors. Surg Endosc. 2012; 26:759–763.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Endoscopic Ultrasound in Gastroenteropancreatic Neuroendocrine Tumors
- Neuroendocrine Tumors of the Female Reproductive Tract: A Literature Review
- Endoscopic Treatment of Gastric and Duodenal Neuroendocrine Tumors: Present and Future
- Two Cases of Endoscopic Papillectomy for Neuroendocrine Tumor Arising from Minor Papilla
- Childhood Neuroendocrine Tumors of Appendix: Suggested Approach and Management